Request a demo specialized to your need.
Introduction:
A virtual clinical trial (also known as remote or decentralized clinical trials) in the definition refers to digitally empowered clinical trial processes. This is a relatively new model of clinical trials in terms of adoption and is, therefore, the highly underutilized method of conducting clinical research.
However, virtual clinical trials have the potential to drive significant digital alterations in clinical research methodologies to create an improved patient-centric ecosystem. These solutions accommodate innumerable benefits of technology, advanced applications, electronic devices, online social engagement platforms, artificial intelligence, robotic process automation, machine learning, among others.
Undoubtedly, virtual clinical trials leverage the power of tele-health/digital technology by including virtual patient monitoring, wearable medical devices, remote SDV, etc. to conduct safe and improved clinical trial research. These virtual trials are patient-centric, cost-effective, and easy to manage.
In terms of market size, the global virtual clinical trial market is estimated to reach US$13.78 bn by 2027, registering a CAGR% of 12.6% for the period 2020-2027 (Source: Polaris Market Research). The growth is driven by the adoption of tele-health, the need for a diverse patient base, solutions for to overcome to overcome time and cost-consuming traditional clinical trial methods, among others.
How is Virtual/Decentralized Clinical Trial Different From the Conventional Trial Process?
Clinical trial research companies believe the use of patient-facing technology helps in widening the pool of trial participants/volunteers, extended patient retention, better data quality, and a holistic patient-centric experience.
It is also believed that virtual clinical trial solutions can significantly improve site staff recruitment, patient recruitment campaigns, safety monitoring, patient education, pre-screening, and data verifications.
In terms of key benefits, cost efficiency is significant with virtual clinical trials and is expected to have a positive impact on the bottom line figures of the pharmaceutical companies. Implementation of these decentralized and remote solutions also enables better time-to-market for new drugs by reducing the overall time period of the clinical trial lifecycle. It also derives benefits through reduced enrollment periods, faster study initiations, lowered patient dropouts, and improved data collection features.
In several cases, it is reported that the overall cost of conducting virtual clinical trials is lower than the conventional clinical trial methods due to remote visit set-ups. This in turn offers capital savings due to reduced number of operational clinical sites. Further cost-benefit is extracted from reduced patient travel reimbursements.
Comparative Analysis: Traditional and Virtual Clinical Trial Process
Traditional Clinical Trial
Virtual Clinical Trial

(Source: MDPI Report)
What are the Technical Capabilities Offered Under the Virtual Clinical Trial Portfolio?
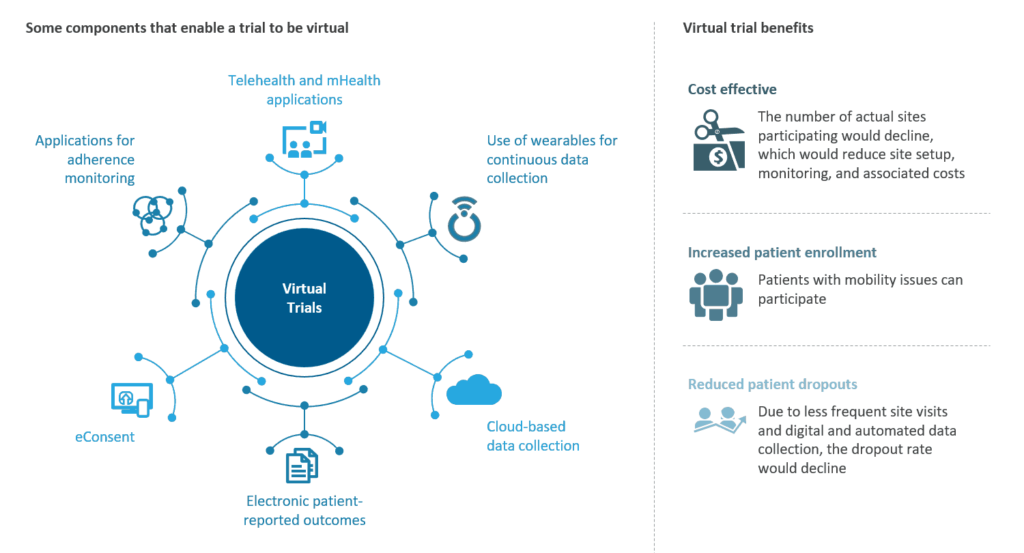
Clinical trial solution providers offer a range of decentralized solutions to cater to different trial requirements across the trial lifecycle. With the help of digitalization and technological advancements, virtual clinical trials have evolved significantly in the last few years. Following are some of the technological capabilities currently employed under decentralized clinical trials:
Following are some of the technological capabilities currently employed under decentralized clinical trials:
- Easy to Use, Locked-Down Tablet HUB, IVR or BYOD
- Program-Centric Bluetooth Peripherals to be used by patients and investigators
- Live video conferencing with Patients/Providers for immediate query solving
- Daily vital captures from mobile phones/ medical wearable devices and self-reported outcomes
- Actionable data and threshold monitoring
- Electronic visit verification
- EMR integration
- ERP Integration
- Gateway and sensor development
- SFTP and ETL services
- Cellular, Wi-Fi, or POTS connectivity
- HL7 enrolment/workflow
- Artificial intelligence for appointment scheduling
What are the Benefits of Virtual Clinical Trial Solutions/ Decentralized Clinical Trial Solutions?
- Informed patients: Helps patients to become more informed and aware of their condition through digitally available study websites and consent portals. Patients are educated thoroughly under the virtual clinical trial procedure. This encourages change in patient engagement methods, quick solution to patient queries and concerns and prompt virtual visits whenever required.
- Improve data quality: Since the data is electronically captured and is closely monitored, the chances for adverse events in clinical trials are minimalistic. With this real-time data sharing, CROs can learn more about patient lifestyle and can implement a patient-centric approach.
- Better communication: Real-time collaboration and communication benefits both the CROs and the patients in transparent information sharing. This results in an effective clinical trial process with optimal results and no miscommunication.
Significant Cost Savings:
- Cost-effective: Virtual visits eliminate the patients’ travel and transportation expenses. In terms of costing, virtual visits eliminate the cost of missed appointments and potential re-admissions.
- Lowered care cost: Virtual clinical trials eliminate hospitalization charges, unnecessary appointment scheduling cost, and much more.
- Increased patient retention: Personalised virtual care ensures better patient retention as availability of investigators and expert opinion is available whenever and wherever required
How do Virtual Clinical Trials Add Value to Clinical Trials?
Virtual/remote/decentralized clinical trial solutions deliver value across all the phases of the clinical trial life cycle and the involved processes. The benefits being innumerable, business enterprises looking forward to adapting or upgrading their clinical trial methodologies are advised to critically consider all the parameters for vendor shortlisting. Read our blog on ‘4 Things to Consider Before Setting Up a Virtual Clinical Trial’.
The major benefit of virtual clinical trials has been reported in patient recruitment and patient monitoring in clinical trials.
How do Decentralized Clinical Trial Solutions Help Patient Recruitment?
According to reports, patient recruitment is the single biggest factor for clinical delays, followed by significant patient fall-outs. It is reported that ~30% of phase 3 study terminations are due to patient enrolment challenges. These delays can cost up to ~US$8mn/day loss for pharmaceutical companies/sponsors.
The conventional patient recruitment methodology involves subject recruitment through hospital visits, medical clinics, or other modes of communication like newspaper/media, etc. The reach is also limited by geographies. However, in virtual clinical trials, an end-to-end digital or hybrid model of patient recruitment is employed. This helps in reaching the enrolment target easily and rapidly making the entire process easy, engaging, and multi-gadget configurable.
This virtual clinical trial patient recruitment approach targets patients directly via web-based platforms and social media sites. This further demolishes any constraints related to geography, race or sex and encourages a better diverse subject population for clinical trial study.
(source: The Everest Group)
What is Remote Patient Monitoring in Virtual Clinical Trials?
Patient monitoring is the core and extremely crucial element of clinical trials. Unlike the conventional methodologies of on-site/face-to-face patient monitoring, remote patient monitoring uses several devices and a virtual interaction module to monitor the patient’s behavior, medical status, and drug efficacy results.
Remote patient monitoring, especially in the digital era with limited on-site engagement options, has witnessed a rapid adoption across CROs, sponsors, and sites for being patient-centric. Virtual monitoring has been reported to reduce the operational cost of clinical trials, deliver improved patient engagement, better patient recovery rates, real-time tracking, and risk analysis, adverse event and protocol deviation monitoring, among others.
According to a study conducted at Duke Clinical Research Institute (DCRI), virtual patient interactions provide value in 5 key areas of clinical trials:
- Passive reporting: Virtual interactions enable patients to immediately follow-up and register responses. This prompt data entry facility reduces any chance of data loss or the need to remember every minuscule information by patients.
- Convenience cost: Sponsors and investigators benefit from a reduced number of patient visits, the enlarged scope of more patient monitoring, reduced staff requirements,
- Big data: Investigators benefit from the increased availability of data from integrated medical devices and mobile applications. The clinical trial process efficacy is leveraged with a real-time heartbeat and SPO2 checks and manual feed in by patients over the portal
- Tracking: Decentralized clinical trial solution enables tracking of every subject related data, clinical visits, medical data, cost per subject, etc. on a real-time basis
- Support: Almost every virtual clinical trial requirement is converted into a cloud-based SaaS solution. These solutions enable multi-gadget configurability (including smartphones) which makes it super convenient for patients, investigators, and CROs to interact from anywhere, anytime, and any device.
Why is Virtual Clinical Trial Considered the Future of Clinical Trials?
Virtual clinical trials as a concept are in the early phase of their adoption and therefore still have several challenges that need to be resolved. However, the positive aspects for the patients, sponsors, CROs, and other stakeholders are relatively high in terms of process simplification and time and cost efficiencies.
The maximum benefit of virtual trials is reaped in phase II and phase IV of the clinical trials. It also delivers value to the pharmaceutical industry by eliminating manual data entries, loss of data during manual data portability, delayed approvals and site payments, loss of time-sensitive patient data, paper-based consent, automated alerts, and file download for submissions and file management, among others.
In coming years, the virtual trial solutions are likely to accommodate advanced versions of artificial intelligence, Robotic Process Automation (RPA), and machine learning to offer even more engaging virtual patient interactions in clinical trials. The outlook is absolutely promising.
With this modern-day technological adaptation, some challenges have also paved their way to this program, such as data privacy, data management, data quality, and others that will be eradicated one by one.
And with this, a new era of virtual trials has already begun to show its potential! Watch our video to gain an insight into virtual clinical trial challenges and solutions to overcome them.
Subscribe to our Newsletter

