Request a demo specialized to your need.
Clinical trials are complex and resource-intensive endeavors. Therefore, there is an increasing need to streamline clinical trial operations and increase operational efficiency. One solution to address this issue is to integrate multiple clinical trial management systems into a unified platform. This approach can be a game-changer in improving operational efficiency in clinical trials. In this analysis report, we will explore how integrating CTMS, Clinical Trials Financials, eTMF, EDC, and Safety in one unified platform can help improve clinical trial operational efficiency.
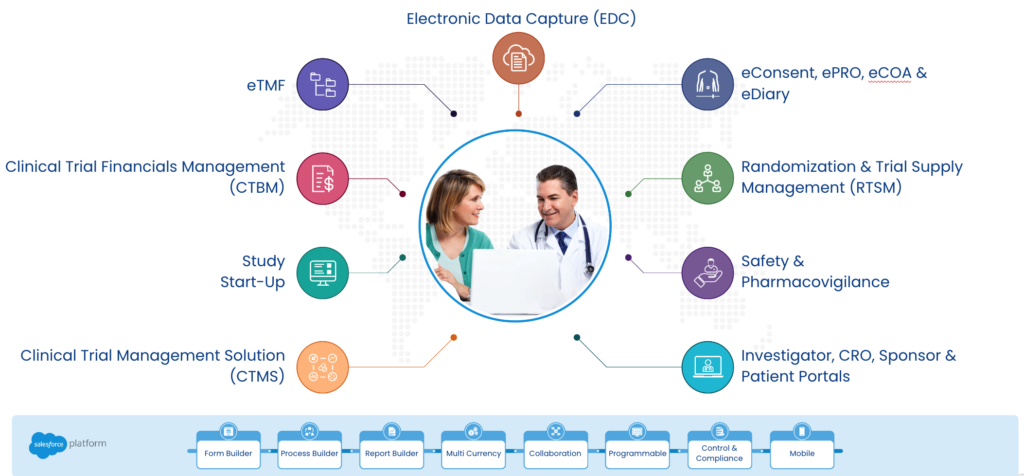
CTMS (Clinical Trial Management System)
A CTMS (Clinical Trial Management System) is a software solution that provides centralized management of clinical trial data and processes. With CTMS, clinical trial teams can streamline clinical trial processes, from study planning to site selection, enrollment, monitoring, and data analysis. It provides real-time tracking of study progress, enabling clinical trial teams to make informed decisions quickly. By integrating CTMS into a unified platform, study teams can have a single source of truth for study data, eliminating the need for manual data entry and reducing the risk of errors.
Clinical Trials Financials
A CTMS (Clinical Trial Management System) is a software solution that provides centralized management of clinical trial data and processes. With CTMS, clinical trial teams can streamline clinical trial processes, from study planning to site selection, enrollment, monitoring, and data analysis. It provides real-time tracking of study progress, enabling clinical trial teams to make informed decisions quickly. By integrating CTMS into a unified platform, study teams can have a single source of truth for study data, eliminating the need for manual data entry and reducing the risk of errors.
eTMF (Electronic Trial Master File)
An Electronic Trial Master File (eTMF) is a digital version of the traditional paper-based Trial Master File (TMF) that contains essential documents and records related to a clinical trial. Integration of eTMF into a unified platform will enable clinical trial teams to have a single, centralized repository for all essential documents, allowing for easy access and sharing of information. This can reduce the time and effort required for document management, improve accuracy, and enable efficient collaboration among study teams.
EDC (Electronic Data Capture)
Electronic Data Capture (EDC) is a software solution used to collect, manage, and store clinical trial data electronically. EDC allows for real-time data collection, validation, and monitoring, reducing the need for manual data entry and paper-based processes. By integrating EDC into a unified platform, real-time data exchange between different clinical trial systems can be enabled, eliminating the need for data reconciliation and reducing data entry errors.
Safety Management
Ensuring patient safety is of paramount importance in clinical trials. Safety management systems are designed to identify and manage adverse events (AEs) and serious adverse events (SAEs) that occur during a clinical trial. Integrating safety management into a unified platform will enable real-time tracking of safety data, including AE and SAE reporting, allowing clinical trial teams to identify and manage safety issues quickly and effectively.
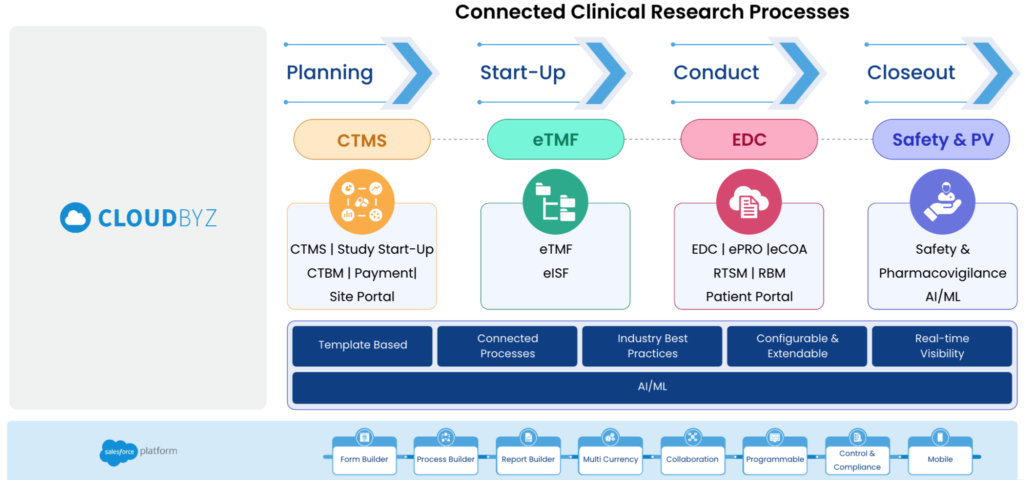
Benefits of Integrating CTMS, Clinical Trials Financials, eTMF, EDC, and Safety into One Unified Platform
Integrating CTMS, Clinical Trials Financials, eTMF, EDC, and Safety into one unified platform can have numerous benefits that can significantly improve the operational efficiency of clinical trials. In this section, we will provide a more detailed analysis of these benefits:
- Improved study management
The integration of various clinical trial systems into a unified platform can improve study management by providing a single source of truth for study data and processes. This can reduce the time and effort required for data entry and management, enabling study teams to focus on more critical tasks.
A unified platform that integrates CTMS, Clinical Trials Financials, eTMF, EDC, and Safety can provide a single source of truth for clinical trial data and processes. This can help clinical trial teams to more efficiently manage study timelines, monitor site performance, and track patient enrollment. With a unified platform, study teams can also more easily track site budgets, study expenses, and vendor payments, allowing for more effective financial management of clinical trials.
- Enhanced data accuracy
Data accuracy is critical in clinical trials, and manual data entry can lead to errors and discrepancies in study data. Integrating EDC into a unified platform can help to reduce the risk of data entry errors by eliminating the need for manual data entry. This can result in more accurate and reliable data, leading to more robust study results.
- Improved data integration and sharing
Integrating different clinical trial systems into one unified platform can improve the integration and sharing of data between different study teams and stakeholders. This can enable faster decision-making and help to reduce delays in study timelines. With a unified platform, study teams can also more easily monitor study progress and identify potential issues in real-time.
- Better collaboration
Collaboration is critical in clinical trials, and a unified platform can help to facilitate collaboration between different study teams and stakeholders. With a unified platform, clinical trial teams can more easily access essential study data and documents, enabling better communication and collaboration between study teams. This can help to reduce delays, improve study quality, and increase operational efficiency.
- Increased cost-effectiveness
Clinical trials are costly, and operational inefficiencies can result in additional expenses. Integrating different clinical trial systems into one unified platform can help to reduce operational inefficiencies and save costs. With a unified platform, study teams can more easily track site budgets, study expenses, and vendor payments, leading to more effective financial management of clinical trials.
- Improved compliance and regulatory oversight
Regulatory compliance is essential in clinical trials, and non-compliance can lead to costly delays and reputational damage. Integrating eTMF into a unified platform can help to ensure compliance with regulatory requirements by providing a centralized repository for all essential documents and records. This can help to reduce the risk of non-compliance, leading to more efficient study timelines and increased operational efficiency.
- Increased operational efficiency
The integration of different clinical trial systems into a unified platform can help streamline clinical trial processes, reducing the risk of errors and improving data accuracy. This can result in increased operational efficiency, faster study timelines, and lower costs.
In conclusion, integrating CTMS, Clinical Trials Financials, eTMF, EDC, and Safety into one unified platform can have numerous benefits that can significantly improve the operational efficiency of clinical trials. With a unified platform, clinical trial teams can more efficiently manage study timelines, monitor site performance, and track patient enrollment, leading to more accurate data, better collaboration, and increased cost-effectiveness.
Cloudbyz Unified Clinical Trial Management (CTMS) is a comprehensive, integrated solution to streamline clinical trial operations. Built on the Salesforce cloud platform, our CTMS provides real-time visibility and analytics across study planning, budgeting, start-up, study management, and close-out. Cloudbyz CTMS can help you achieve greater efficiency, compliance, and quality in your clinical operations with features like automated workflows, centralized data management, and seamless collaboration. Contact us today to learn how Cloudbyz CTMS can help your organization optimize its clinical trial management processes.
To know more about the Cloudbyz Unified Clinical Trial Management Solution contact info@cloudbyz.com
Subscribe to our Newsletter

