Request a demo specialized to your need.

Clinical trials are crucial in advancing medical research and finding new treatments and cures for various diseases. However, the process of managing clinical trials is complex and time-consuming, requiring significant financial and human resources. The economic slowdown has had a significant impact on healthcare organizations, making it imperative to find ways to reduce costs while maintaining the quality of research. One solution is the implementation of a unified clinical trial management platform. In this blog, we will explore how this platform can help save costs and improve efficiency during an economic slowdown.
What is a Unified Clinical Trial Management Platform?
A unified clinical trial management platform is a comprehensive software system designed to manage all aspects of clinical trials. This platform includes features such as patient recruitment, data management, regulatory compliance, and study monitoring. The platform is designed to be user-friendly, allowing researchers to easily manage and monitor their studies.
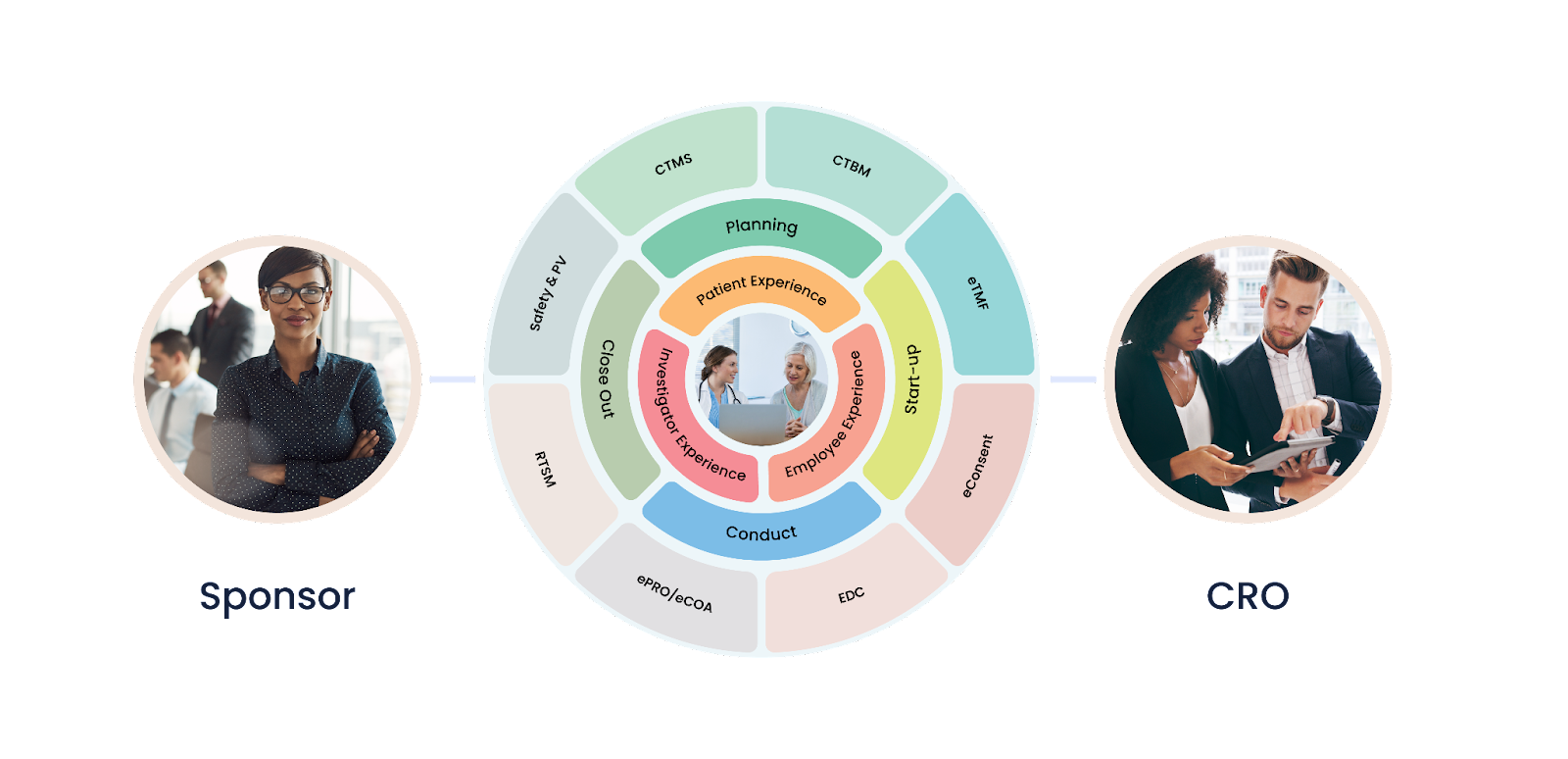
How Does a Unified Clinical Trial Management Platform Help Save Costs?
- Reduces Administrative Burden
Clinical trials involve a significant amount of administrative work, including data entry, regulatory compliance, and patient management. A unified clinical trial management platform automates many of these tasks, reducing the administrative burden and allowing staff to focus on more critical tasks, such as patient care and study design.
- Reduces Resource Costs
Clinical trials require a significant investment of resources, including staff time, equipment, and facilities. A unified clinical trial management platform can help reduce resource costs by optimizing resource utilization. For example, the platform can help researchers identify available equipment and facilities, reducing the need for additional resources.
- Reduces Monitoring Costs
Clinical trial monitoring is essential to ensure patient safety and data quality. However, monitoring can be costly, requiring staff to travel to study sites and conduct on-site visits. A unified clinical trial management platform can reduce monitoring costs by providing real-time data access and remote monitoring capabilities. This reduces the need for on-site visits, reducing travel costs and increasing efficiency.
How Does a Unified Clinical Trial Management Platform Improve Efficiency?
- Streamlines Processes
Clinical trials involve many different processes, including patient recruitment, data management, and regulatory compliance. A unified clinical trial management platform streamlines these processes, reducing the time and effort required to manage clinical trials. This allows researchers to focus on study design and patient care, improving overall efficiency.
- Improves Collaboration
Clinical trials often involve multiple stakeholders, including researchers, clinicians, regulatory bodies, and patients. A unified clinical trial management platform improves collaboration by providing a centralized platform for communication and data sharing. This reduces communication errors and delays, improving overall efficiency.
- Enhances Data Quality
Data quality is critical in clinical trials, as it affects the validity and reliability of study results. A unified clinical trial management platform enhances data quality by providing real-time data access and reducing data entry errors. This improves the accuracy and completeness of study data, improving overall efficiency.
Conclusion
In conclusion, a unified clinical trial management platform can help healthcare organizations save costs and improve efficiency during an economic slowdown. By reducing administrative burden, resource costs, and monitoring costs, the platform allows researchers to focus on study design and patient care. Additionally, the platform streamlines processes improve collaboration, and enhances data quality, improving overall efficiency. As such, a unified clinical trial management platform is an essential tool for healthcare organizations looking to conduct clinical trials in a cost-effective and efficient manner.
Cloudbyz Unified Clinical Trial Management (CTMS) is a comprehensive, integrated solution to streamline clinical trial operations. Built on the Salesforce cloud platform, our CTMS provides real-time visibility and analytics across study planning, budgeting, start-up, study management, and close-out. Cloudbyz CTMS can help you achieve greater efficiency, compliance, and quality in your clinical operations with features like automated workflows, centralized data management, and seamless collaboration. Contact us today to learn how Cloudbyz CTMS can help your organization optimize its clinical trial management processes.
To know more about the Cloudbyz Unified Clinical Trial Management Solution contact info@cloudbyz.com
Subscribe to our Newsletter

