Coverage analysis is a critical component of clinical trial budgeting, ensuring compliance with Centers for Medicare & Medicaid Services (CMS) guidelines and determining which study-related procedures are billable to insurance and which are the sponsor’s responsibility. It is an essential process to maintain both financial accuracy and regulatory compliance in clinical research.
At its core, coverage analysis requires mapping each procedure in a clinical trial to either Standard of Care (SoC)—which can be billed to insurance—or sponsor-specific costs. This process directly influences budget accuracy, contract negotiations, and site reimbursement.
The Cloudbyz Advantage in Coverage Analysis
Cloudbyz Clinical Trial Financial Management (CTFM) solution is designed to streamline and strengthen the coverage analysis process through automation, standardization, and real-time insights. Here's how:
1. Standardized Procedure Library with SoC Designation
Cloudbyz CTFM comes with a built-in library of standard clinical procedures. Each procedure can be pre-designated as Standard of Care, allowing for quick and consistent classification during study setup. This eliminates manual tracking and reduces the risk of misclassification, which can otherwise lead to billing errors or regulatory non-compliance.
2. Automated Inheritance of SoC Status in Study Setup
When a study’s Schedule of Events or Visit Plan is being defined, any procedure added from the library automatically carries forward its SoC designation. This inheritance capability ensures consistent application of coverage determinations across studies, reducing administrative burden and increasing accuracy. Additionally, users retain the flexibility to override or flag procedures as SoC at the study level if needed, giving teams control without sacrificing consistency.
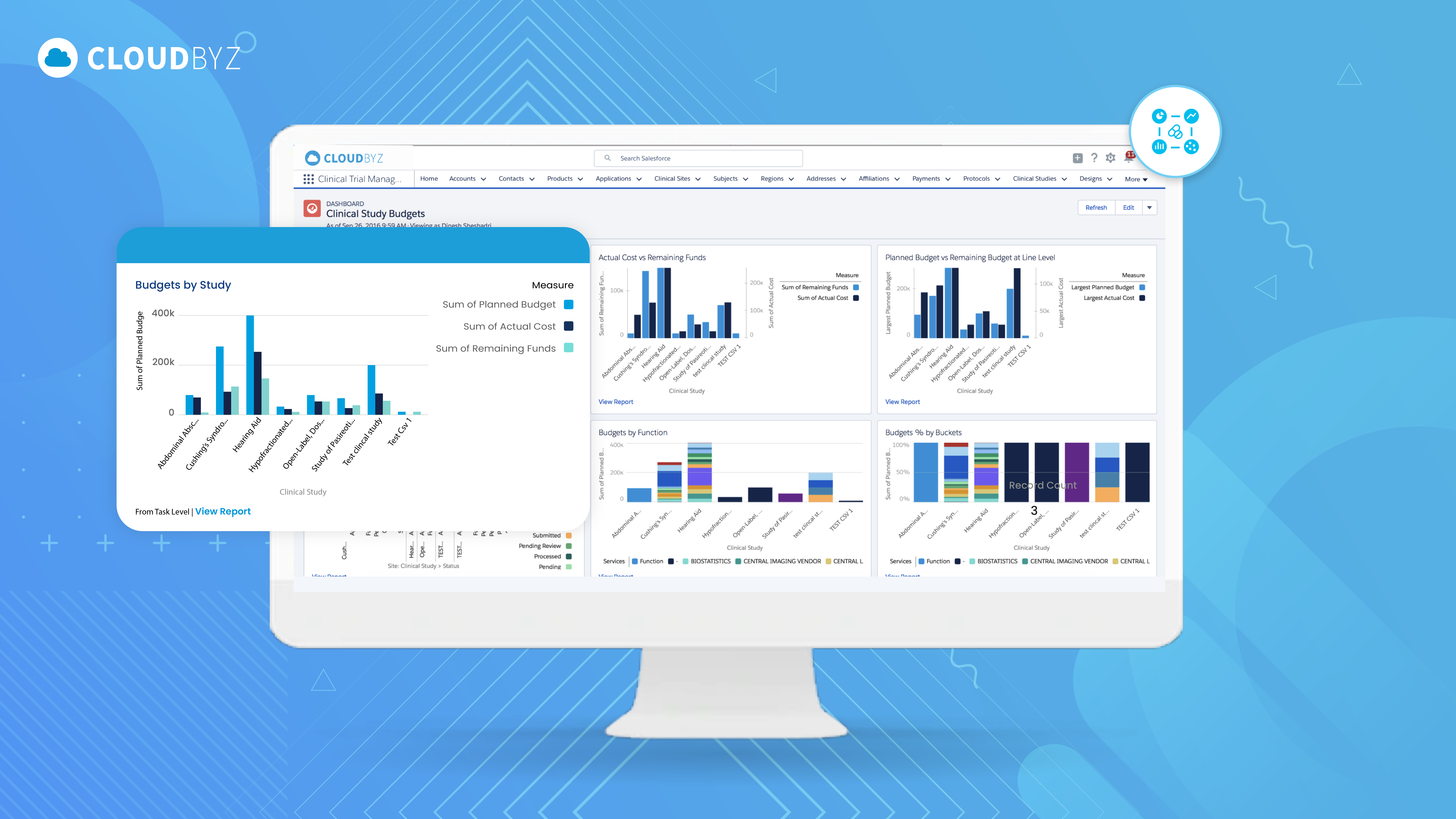
3. Cross-Study Visibility & Reporting
One of the most powerful features of Cloudbyz CTFM is its robust reporting and analytics dashboard. Users can generate real-time reports on SoC versus non-SoC procedures within a study or across a portfolio of studies. This allows sponsors and research organizations to:
-
Track insurance-billable activities
-
Ensure alignment with Medicare guidelines
-
Support audit readiness
-
Identify trends and opportunities for financial optimization
4. Informed Budgeting and Sponsor Negotiations
By integrating coverage analysis directly into the budgeting workflow, Cloudbyz helps users build accurate, defensible budgets. The system clearly separates reimbursable and sponsor-covered costs, enabling transparent budget discussions with both sponsors and investigative sites. This leads to faster budget approvals and fewer downstream billing issues.
5. Regulatory Compliance and Risk Reduction
Through standardized processes, automated SoC tracking, and full audit trails, Cloudbyz CTFM supports compliance with CMS regulations and other billing oversight requirements. This reduces the risk of overbilling payers or under-budgeting trials—both of which carry financial and legal risk.
Conclusion
Coverage analysis is no longer a manual, error-prone process. With Cloudbyz CTFM, clinical research sponsors and sites gain an intelligent, integrated toolset to manage coverage analysis with confidence. From automated SoC classification to portfolio-wide coverage insights, Cloudbyz enables accurate budgeting, transparent sponsor negotiations, and streamlined compliance—all within a unified clinical trial management ecosystem.