Request a demo specialized to your need.

Pharmacovigilance is a crucial aspect of the healthcare industry, as it involves the detection, assessment, understanding, and prevention of adverse effects associated with the use of medications. Pharmacovigilance solutions are designed to support these activities and help ensure patient safety. In this blog, we will discuss the must-have business, functional and technical capabilities in a pharmacovigilance solution.
Business Capabilities
- Case Management: The pharmacovigilance solution must have a robust case management system that can handle the entire lifecycle of a case, from initial receipt to final closure. The system should be able to manage all case-related information, including patient demographics, medication details, adverse event information, and follow-up information.
- Signal Detection and Management: The solution should be able to detect and manage signals or patterns that indicate potential adverse events. It should also have the capability to generate alerts and notifications for further investigation.
- Regulatory Reporting: The solution must support regulatory reporting requirements, including the ability to generate periodic safety reports (PSURs) and expedited reports to regulatory authorities. It should also have the capability to track and manage reportable events and communicate with regulatory agencies.
- Risk Management: The solution should be able to manage risks associated with medications and help organizations develop risk management plans to mitigate those risks. It should also have the capability to monitor and evaluate the effectiveness of risk management plans.
- Data Management and Analysis: The pharmacovigilance solution must have the capability to manage large amounts of data and provide real-time analytics and reporting. It should also have the ability to identify trends and patterns in adverse events and generate actionable insights.
Functional Capabilities
- Data Capture: The solution should be able to capture data from multiple sources, including clinical trials, social media, and electronic health records. It should also have the capability to validate data and ensure data quality.
- Coding and Classification: The solution must support coding and classification of adverse events according to established standards such as MedDRA and WHO-ART. It should also have the capability to map different coding systems to ensure consistency across the organization.
- Workflow Management: The solution must have the capability to manage workflows and automate processes, including case processing, signal detection, and report generation. It should also have the ability to track and manage tasks and activities.
- Collaboration and Communication: The pharmacovigilance solution must support collaboration and communication among team members, including the ability to share data and documents securely. It should also have the capability to communicate with external stakeholders such as healthcare providers, regulatory agencies, and patients.
- System Integration: The solution must have the capability to integrate with other systems such as electronic health records, clinical trial systems, and regulatory databases. It should also have the ability to exchange data with external systems using standard formats such as XML and HL7.
Technical Capabilities
A modern pharmacovigilance solution must have the following technical capabilities to effectively monitor, detect, assess, and prevent adverse events related to the use of pharmaceutical products:
- Data integration: The solution should seamlessly integrate data from multiple sources, such as electronic health records, spontaneous reporting systems, social media, literature, and clinical trials, to provide a comprehensive view of drug safety information.
- Artificial intelligence and machine learning: Advanced algorithms should be employed to automate data mining, signal detection, and analysis of potential safety issues, helping to reduce manual workload and improve efficiency.
- Natural language processing (NLP): The system should be able to analyze and extract relevant information from unstructured data, such as patient narratives and medical literature, allowing for a more efficient and accurate assessment of adverse events.
- Real-time monitoring and analytics: The solution should enable continuous monitoring of drug safety data and provide real-time analytics to support proactive decision-making and timely interventions.
- Advanced signal detection and management: The system should be able to identify and assess potential safety signals, prioritize them based on their severity and likelihood, and facilitate appropriate follow-up actions.
- Risk management and mitigation: The solution should support the development and implementation of risk management plans, including the identification of risk factors, the assessment of their impact, and the implementation of risk mitigation strategies.
- Regulatory compliance: The solution should ensure adherence to global and regional pharmacovigilance regulations, such as ICH guidelines, FDA requirements, and EMA regulations, and support the generation of periodic safety update reports (PSURs) and other regulatory submissions.
- Scalability and adaptability: The system should be able to scale up and adapt to growing data volumes and changing regulatory requirements, ensuring long-term sustainability and effectiveness.
- Security and data privacy: The solution should ensure that sensitive patient data and confidential information are securely stored and transmitted in accordance with data protection regulations, such as GDPR and HIPAA.
- Collaboration and knowledge sharing: The solution should facilitate seamless collaboration and knowledge sharing among different stakeholders, including pharmaceutical companies, regulatory agencies, healthcare professionals, and patients.
- User-friendly interface: The system should have an intuitive, user-friendly interface that allows for easy navigation and efficient management of pharmacovigilance activities.
- Customizable reporting and visualization: The solution should provide customizable reporting and data visualization tools to help users analyze and interpret complex safety data and make informed decisions.

In conclusion, a pharmacovigilance solution must have a wide range of business, functional and technical capabilities to support the complex activities involved in ensuring patient safety. Organizations must carefully evaluate potential solutions to ensure they meet their specific needs and requirements. By choosing a robust and comprehensive pharmacovigilance solution, organizations can enhance their ability to detect and manage adverse events, comply with regulatory requirements, and ultimately, improve patient outcomes.
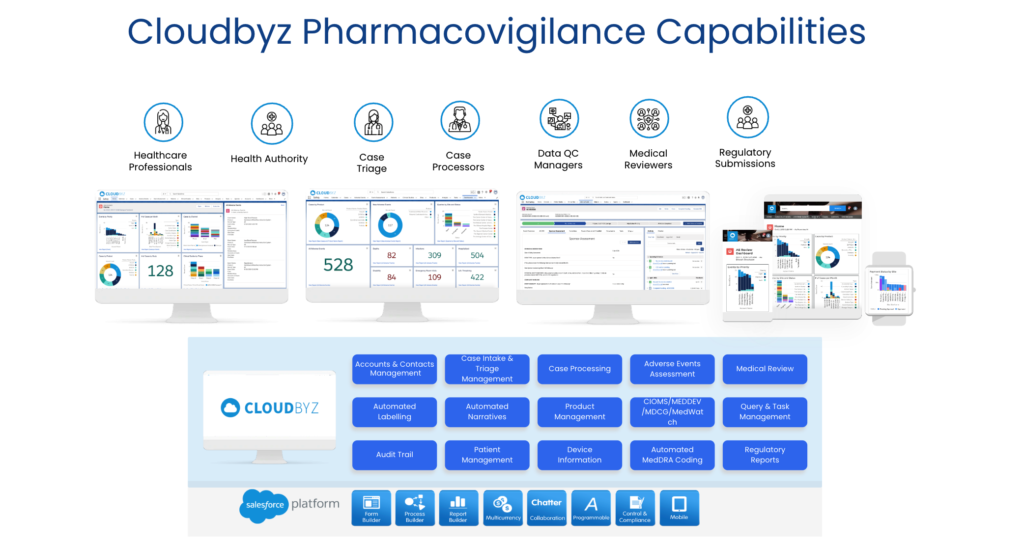
Cloudbyz Safety and Pharmacovigilance (PV) software is a cloud-based solution built natively on the Salesforce platform. It offers 360 degree view across R&D and commercial. It also enables pharma, bio-tech and medical devices companies to make faster and better safety decisions. It helps to optimize global pharmacovigilance compliance along with easy to integrate risk management features. Cloudbyz pharmacovigilance software solution easily integrates the required data over a centralized cloud-based platform for advanced analytics set-up along with data integrity. It empowers the end-user with proactive pharmacovigilance, smart features with data-backed predictability, scalability and cost-effective support.
To know more about Cloudbyz safety & pharmacovigilance contact info@cloudbyz.com
Subscribe to our Newsletter

