Request a demo specialized to your need.
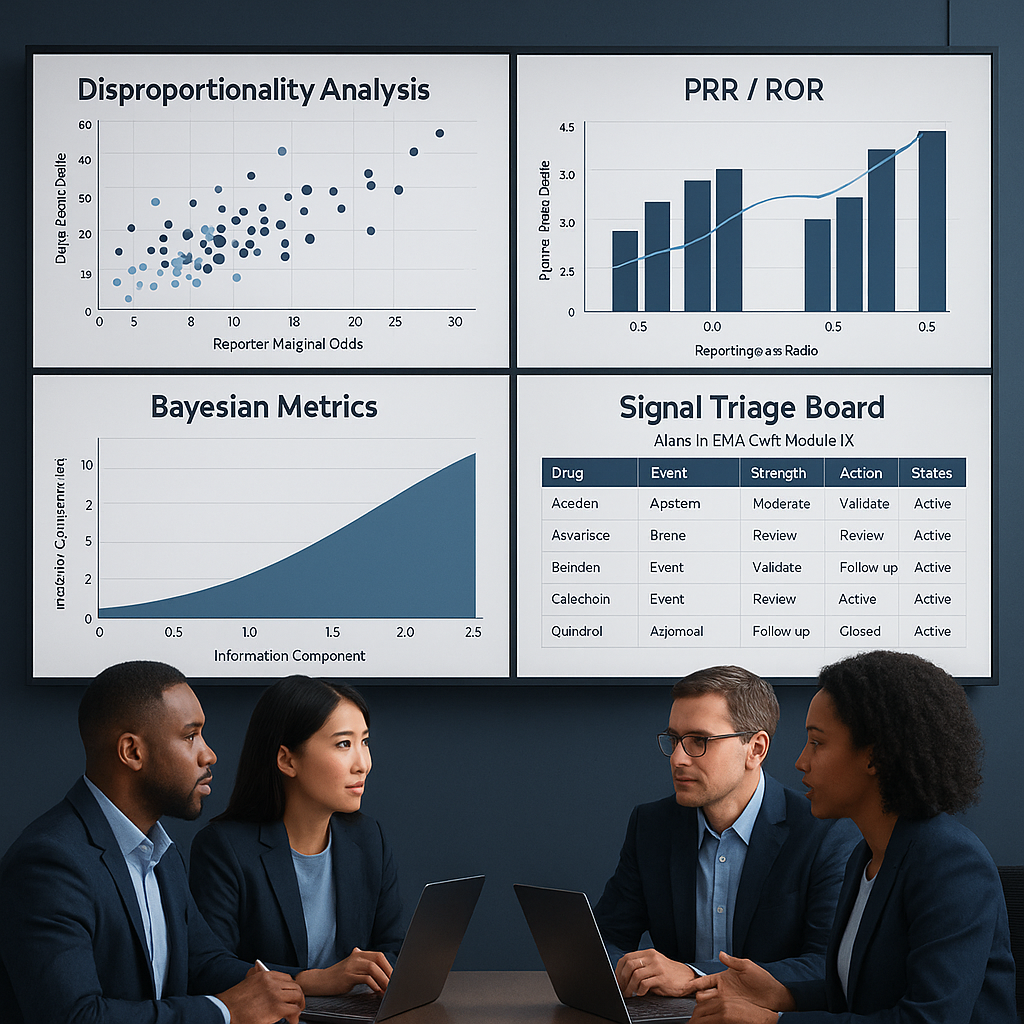
Turn EMA GVP IX into daily, risk-based signal detection and triage.
From Algorithms to Insight: Building a Risk-Based Signal Detection Framework
Signal detection is often discussed in terms of methods—PRRs, RORs, ICs, dashboards, and thresholds. Yet regulators and mature pharmacovigilance organizations know that effective signal detection is not about statistics alone. It is about risk focus, execution discipline, and governance. When grounded in a clear benefit–risk context and operationalized with rigor, signal detection evolves from a compliance obligation into a strategic system for earlier patient protection.
A high-performing framework starts by acknowledging a simple truth: not all products, risks, or data streams deserve equal attention.
Grounding Signal Detection in Risk and Product Context
The cornerstone of a modern signal detection program is a clearly articulated benefit–risk context for each product. This begins with defining critical-to-quality (CTQ) safety factors—patient populations, known class effects, vulnerable cohorts, routes of administration, and exposure profiles—that truly matter for patient outcomes. Methods, thresholds, and cadences should then be selected to reflect those risks, not applied uniformly across the portfolio.
Disproportionality analysis on spontaneous reporting data remains a foundational tool, but it is insufficient on its own. High-value programs complement statistical screening with targeted literature surveillance, clinical trial data review, solicited program outputs, and real-world evidence where appropriate. This multi-source approach ensures that emerging risks are contextualized rather than treated as isolated numerical signals.
Data quality must be addressed before any algorithm runs. Robust ingestion controls—duplicate detection, date plausibility checks, product and indication coding validation, and reporter classification—prevent noisy inputs from overwhelming signal engines and reviewers. Stratification logic (by age, sex, region, dose, or formulation) and masking strategies for very common events should be explicitly defined and documented to avoid inconsistent interpretations.
Detection frequency should scale with risk and exposure. High-volume products or therapies with narrow therapeutic windows warrant tighter scanning intervals and faster triage service levels, while lower-risk products may justify broader cadences. Initial thresholds should be anchored in historical baselines and refined through calibration sprints, with every adjustment versioned and justified so reviews remain reproducible over time.
Clear ownership reinforces integrity. Biostatisticians are responsible for methodological soundness, safety physicians for medical plausibility, and pharmacovigilance operations for workflow execution and evidence capture. Encoding these responsibilities into SOPs and operating manuals ensures consistency even as teams grow or change. Regulatory expectations and terminology should be anchored in authoritative sources, with European Medicines Agency guidance—specifically GVP Module IX—setting the baseline vocabulary and structure.
Operationalizing Triage, Validation, and Assessment
Frameworks only deliver value through disciplined execution. High-performing organizations operationalize signal management through structured triage and assessment workflows that reduce bias and improve reproducibility.
A centralized triage board should present new signals with a consistent evidence package: statistical outputs, case series summaries, relevant clinical context, and curated literature excerpts. Standardized triage notes are essential, capturing initial medical plausibility, strength of evidence, and preliminary causality thinking in a format that supports later audit and review.
Validation must be distinct from detection. This separation reduces confirmation bias and reinforces methodological integrity. Validation activities typically include focused medical review, case-level drill-downs, and sensitivity analyses such as removing duplicates, re-coding product families, or adjusting stratifications. Only validated signals should proceed to formal assessment.
Assessments themselves should follow predefined questions: biological plausibility, dose–response relationships, time-to-onset patterns, de-challenge and re-challenge evidence, and consistency across data sources. Each assessment should conclude with a clear, documented recommendation—continued monitoring, labeling updates, targeted studies, or closure—with explicit linkage to downstream actions such as risk management plan updates, communications, or regulatory submissions.
Decision trails must be comprehensive. Inspectors increasingly expect organizations to demonstrate exactly which data cuts, MedDRA versions, and analytical parameters were used at the time a decision was made. Automation can scale detection and visualization, but human judgment remains essential. Well-designed dashboards surface exceptions—such as sudden increases in pediatric risk—while enabling rapid drill-down to individual case narratives. Templates and playbooks help onboard new reviewers without diluting standards.
Closing the Loop with Governance, Metrics, and Learning
Sustained excellence in signal management depends on strong governance and continuous learning. Leading organizations establish cross-functional safety governance forums that review signal dashboards, aging metrics, and CAPA status on a fixed cadence. These forums reinforce accountability and ensure that emerging risks receive timely senior oversight.
Meaningful metrics go beyond volume counts. Time from detection to triage, validation pass rates, assessment cycle times, and the proportion of signals resulting in labeling or risk-management actions provide insight into both efficiency and effectiveness. Method performance should also be monitored, including false discovery rates, stability across dictionary upgrades, and the behavior of high-risk sub-populations.
Evidence management underpins inspection readiness. An auditable repository that stores statistical outputs, case series, medical rationales, and final decisions—each time-stamped and approved—demonstrates control and transparency. As decisions progress toward regulatory communications, alignment with regional requirements becomes critical. In Europe, GVP Module IX and its methodological addenda remain central references, while global programs must harmonize with international standards such as International Council for Harmonisation guidance on case quality and timeliness.
Finally, learning closes the loop. After major assessments, teams should capture lessons learned and update thresholds, dictionaries, and SOPs where justified. Ongoing training on emerging methodologies and data sources ensures programs stay current without sacrificing rigor.
When designed and executed well, a risk-based signal detection framework transforms regulatory guidance from a checklist into a living system—one that enables earlier risk recognition, clearer decision-making, and stronger protection for patients worldwide.
Subscribe to our Newsletter

