Request a demo specialized to your need.
Pharmacovigilance is the process of monitoring and assessing adverse drug reactions (ADRs) and other medication safety issues. Medical coding is a key aspect of pharmacovigilance, as it involves the systematic classification and indexing of ADRs and other medical events. Medical coding plays a critical role in pharmacovigilance because it enables the accurate and consistent recording and analysis of adverse events, which is essential for identifying patterns, trends, and potential safety risks associated with medication use.
However, the process of medical coding can be time-consuming and labor-intensive, requiring highly trained professionals to manually review and classify medical records and other clinical data. This process can be further complicated by the use of multiple coding systems, which may not always be compatible with one another.
To address these challenges, pharmacovigilance organizations are increasingly turning to automation technologies to streamline and optimize the medical coding process. Pharmacovigilance medical coding automation refers to the use of artificial intelligence (AI) and machine learning (ML) algorithms to automate the medical coding process, improving accuracy, speed, and efficiency.
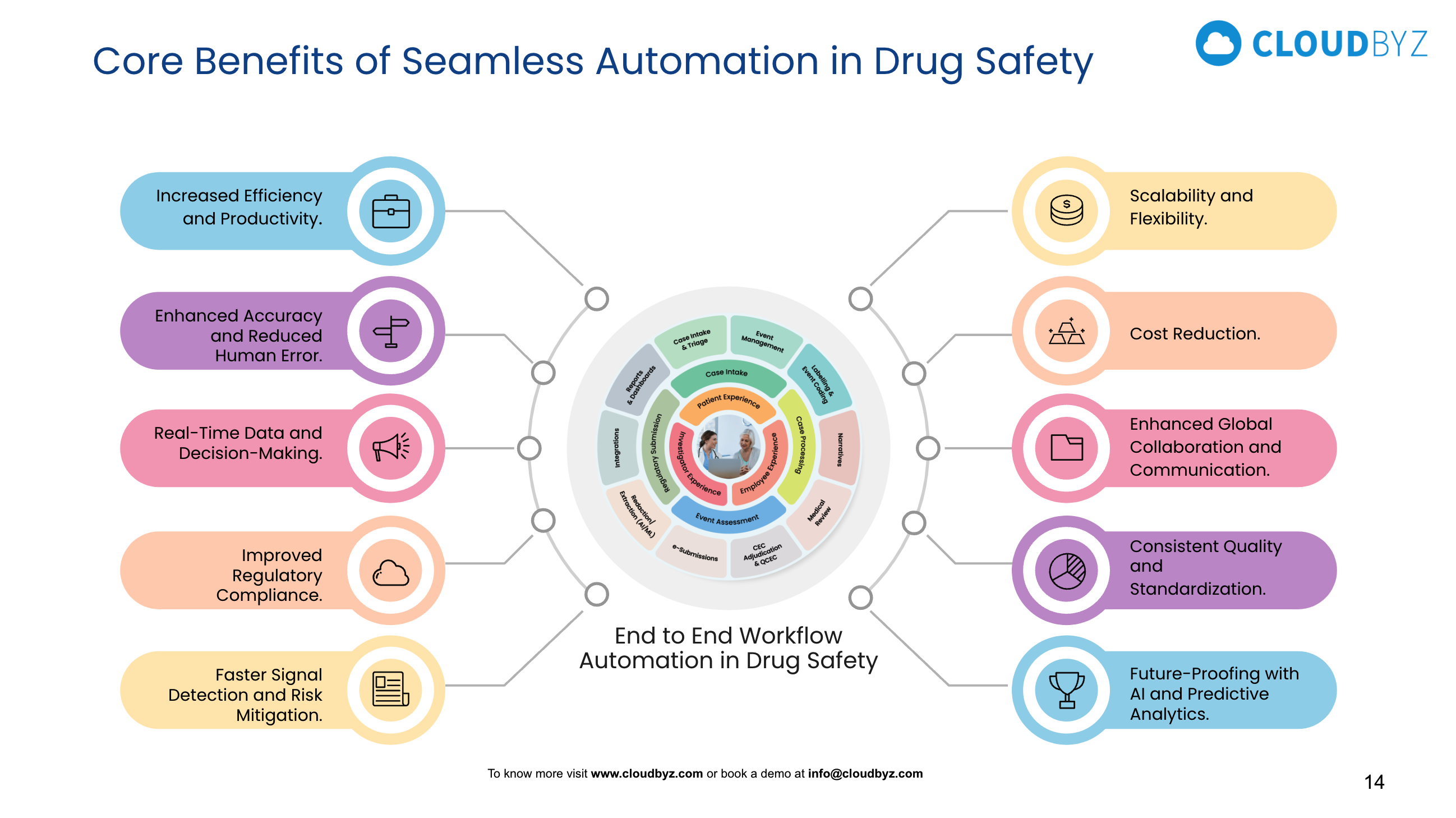
There are several benefits to pharmacovigilance medical coding automation. First, it can significantly reduce the time and resources required to manually code medical data, enabling pharmacovigilance professionals to focus on more complex and critical tasks. Additionally, automation can improve the accuracy and consistency of medical coding, reducing the risk of errors and discrepancies in ADR reporting.
Another advantage of pharmacovigilance medical coding automation is that it can help to standardize coding across different regions and languages, allowing for more consistent and reliable global pharmacovigilance data. By using machine learning algorithms to identify patterns and relationships between medical events and specific medications, pharmacovigilance professionals can gain new insights into medication safety and make more informed decisions about drug development and regulation.
However, there are also some challenges and limitations to pharmacovigilance medical coding automation. One key challenge is the need to ensure that automated systems are properly validated and calibrated to accurately reflect human medical coding standards. Additionally, machine learning algorithms may not always capture the full context and nuances of medical data, which can lead to inaccuracies in coding and reporting.
To address these challenges, pharmacovigilance organizations must work to implement rigorous quality control measures and ensure that automated systems are regularly monitored and evaluated for accuracy and performance. This may involve the use of hybrid systems that combine machine learning algorithms with human review and oversight, as well as ongoing training and education for pharmacovigilance professionals on the use of automated coding systems.
Important Considerations
Pharmacovigilance is the science and activities related to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. Medical coding is a key component of pharmacovigilance, as it involves assigning standardized codes to medical terms in order to enable efficient data management and analysis. With the growing volume of pharmacovigilance data, automation of medical coding has become an attractive option. Following are important things to consider for pharmacovigilance medical coding automation.
- Quality of data: The quality of data is crucial for successful medical coding automation. Data quality can be improved by using standardized data sources, validating data accuracy and consistency, and addressing any data quality issues prior to automation.
- Regulatory compliance: Pharmacovigilance is subject to strict regulatory requirements, such as reporting obligations and quality management systems. Therefore, it is important to ensure that any automated coding processes comply with these regulations.
- Language: Medical coding involves assigning codes to medical terms, which can be in different languages. Therefore, it is important to consider the language of the data and ensure that the automated coding system can handle multiple languages.
- Complexity of data: Medical coding can be a complex process, particularly in pharmacovigilance, where data may involve multiple variables and interrelated factors. The automated coding system should be able to handle such complexity and produce accurate and meaningful results.
- Integration with existing systems: Pharmacovigilance involves multiple processes, such as case management, data analysis, and reporting. Therefore, it is important to ensure that the automated coding system can be integrated with existing systems and workflows to enable efficient data management and analysis.
- Expertise: While automation can improve the efficiency and accuracy of medical coding, it is important to have experts involved in the process to ensure quality control and provide input to improve the automation process.
- Validation and testing: The automated coding system should be validated and tested to ensure its accuracy and reliability. This involves comparing the results of the automated coding system with manual coding and ensuring that any discrepancies are addressed.
- Maintenance: The automated coding system should be regularly maintained to ensure its ongoing accuracy and reliability. This involves updating the system to reflect changes in coding standards and regulations, and addressing any issues that arise.
In conclusion, pharmacovigilance medical coding automation is a promising and rapidly evolving field that has the potential to revolutionize the way pharmacovigilance professionals monitor and assess medication safety. By leveraging the power of AI and machine learning, pharmacovigilance organizations can streamline the medical coding process, improve the accuracy and consistency of ADR reporting, and gain new insights into medication safety trends and risks. However, to fully realize the benefits of pharmacovigilance medical coding automation, organizations must invest in robust quality control measures and ongoing training and education for their teams.
Automation of medical coding in pharmacovigilance can offer significant benefits in terms of efficiency and accuracy. However, it is important to consider the quality of data, regulatory compliance, language, complexity of data, integration with existing systems, expertise, validation and testing, and maintenance. By addressing these considerations, the automation of medical coding in pharmacovigilance can enable more efficient and effective management of adverse drug events and improve patient safety.
Cloudbyz Safety and Pharmacovigilance (PV) is a cloud-based solution built natively on the Salesforce platform. It offers 360 degree view across R&D and commercial. It also enables pharma, bio-tech and medical devices companies to make faster and better safety decisions. It also helps to optimize global pharmacovigilance compliance along with easy to integrate risk management features.
Our pharmacovigilance software solution easily integrates the required data over a centralized cloud-based platform for advanced analytics set-up along with data integrity. It empowers the end-user with proactive pharmacovigilance, smart features with data-backed predictability, scalability and cost-effective support.
Subscribe to our Newsletter

