Request a demo specialized to your need.

A unified clinical trial management platform can enable risk-based monitoring (RBM) in clinical trials by providing a centralized platform for data management, monitoring, and reporting.
A unified clinical trial management platform is a cloud-based software solution that integrates all the essential components of clinical trials, such as CTMS, eTMF, EDC, eConsent, ePRP, patient recruitment and safety into a single platform. This platform provides life sciences organizations with a centralized and holistic view of all their clinical trial activities, enabling them to manage their trials more efficiently and effectively.
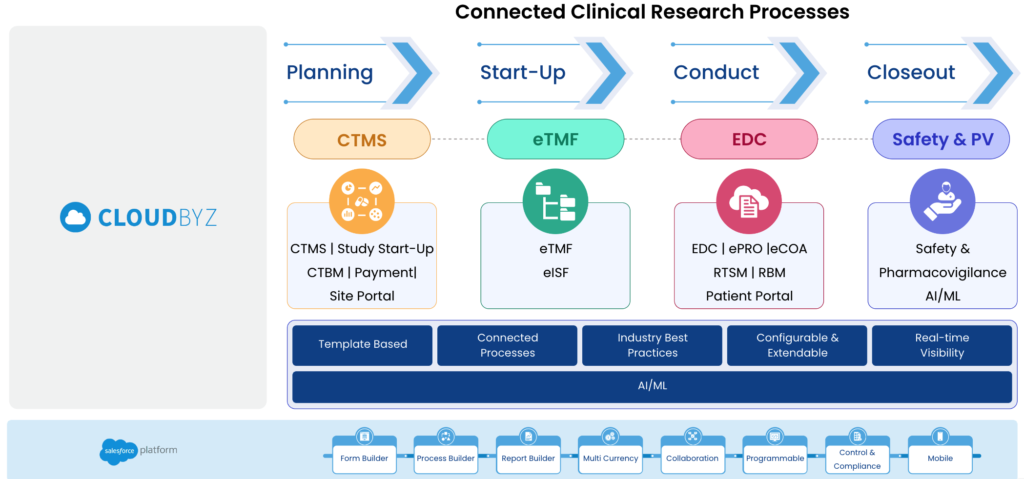
Here are some ways in which a unified clinical trial management platform can enable RBM:
- Risk assessment and monitoring: A unified clinical trial management platform can facilitate the risk assessment process by providing a centralized platform for identifying and tracking potential risks throughout the trial. This can help to ensure that the most critical data points are monitored effectively and that potential risks are identified and addressed proactively.
- Data management: RBM requires a sophisticated data management approach to ensure that data is collected, cleaned, and analyzed effectively. A unified clinical trial management platform can provide a centralized platform for data management, ensuring that data is collected and managed consistently across all trial sites and data sources.
- Remote monitoring: RBM allows for remote monitoring of clinical trial data, reducing the need for on-site monitoring visits. A unified clinical trial management platform can provide a centralized platform for remote monitoring, allowing monitoring teams to access data from any location, and enabling more efficient and effective monitoring activities.
- Real-time data reporting: RBM requires real-time data reporting to ensure that potential issues are identified and addressed proactively. A unified clinical trial management platform can provide real-time data reporting, allowing stakeholders to access and analyze data in real-time, and enabling more efficient and effective decision-making.
- Collaboration and communication: Effective communication and collaboration among stakeholders are essential for RBM success. A unified clinical trial management platform can facilitate collaboration and communication by providing a centralized platform for sharing information, communicating updates, and addressing issues in real-time.
- Customized dashboards and metrics: RBM requires customized dashboards and metrics to ensure that data is monitored effectively. A unified clinical trial management platform can provide customized dashboards and metrics, allowing stakeholders to monitor the most critical data points and identify potential issues proactively.
- Streamlined data integration: RBM requires the integration of data from multiple sources, including electronic data capture (EDC) systems, clinical trial management systems (CTMS), and electronic health records (EHRs). A unified clinical trial management platform can streamline data integration, allowing stakeholders to access and analyze data from multiple sources in a single platform.
- Automated data analytics: RBM requires sophisticated data analytics to ensure that potential risks are identified and addressed proactively. A unified clinical trial management platform can provide automated data analytics, allowing stakeholders to identify patterns, trends, and potential risks more efficiently and effectively.
- Intelligent monitoring: RBM requires intelligent monitoring to ensure that potential issues are identified and addressed proactively. A unified clinical trial management platform can provide intelligent monitoring capabilities, including predictive analytics and machine learning, allowing stakeholders to identify potential risks before they become major issues.
- Quality management: RBM requires a robust quality management approach to ensure that data is collected and managed in a consistent and reliable manner. A unified clinical trial management platform can provide quality management capabilities, including standardized processes and workflows, audit trails, and quality control checks, ensuring that data is collected and managed according to best practices.
- Customizable workflows: RBM requires customizable workflows to ensure that monitoring activities are tailored to the specific trial and risk profile. A unified clinical trial management platform can provide customizable workflows, allowing stakeholders to create customized monitoring plans and activities that are tailored to the specific needs of the trial.
- Mobile access: RBM requires mobile access to ensure that stakeholders can access and analyze data from any location. A unified clinical trial management platform can provide mobile access, allowing stakeholders to access data from any device, anywhere, at any time.
In summary, a unified clinical trial management platform can enable RBM by providing a centralized platform for risk assessment, data management, remote monitoring, real-time data reporting, collaboration and communication, and customized dashboards and metrics, streamlined data integration, automated data analytics, intelligent monitoring, quality management, customizable workflows, and mobile access. By providing these capabilities, a unified clinical trial management platform can help organizations to implement a successful RBM approach, ensuring that their clinical trials are conducted efficiently and effectively.
Cloudbyz Unified Clinical Trial Management (CTMS) is a comprehensive and integrated solution designed to streamline clinical trial operations. Built on the Salesforce cloud platform, our CTMS provides real-time visibility and analytics across study planning, budgeting, start-up, study management, and close-out. With features like automated workflows, centralized data management, and seamless collaboration, Cloudbyz CTMS can help you achieve greater efficiency, compliance, and quality in your clinical operations. Contact us today to learn more about how Cloudbyz CTMS can help your organization optimize its clinical trial management processes.
To know more about Cloudbyz Unified Clinical Trial Management Solution contact info@cloudbyz.com
Subscribe to our Newsletter

