Request a demo specialized to your need.
Subscribe to our Newsletter

Dinesh
In today’s competitive landscape, midsize Contract Research Organizations (CROs) are under constant pressure to stay ahead of the curve by providing quality clinical trial services at competitive prices. In order to achieve this, CROs need to adopt innovative technologies and solutions that can help streamline their operations and improve their efficiency. One such solution is a unified clinical trial management platform that can help midsize CROs deliver services and achieve a competitive advantage.

A unified clinical trial management platform is a cloud-based software solution that integrates all the essential components of clinical trials, such as study design, patient recruitment, data collection, data management, analysis, and reporting, into a single platform. This platform provides CROs with a centralized and holistic view of all their clinical trial activities, enabling them to manage their trials more efficiently and effectively.
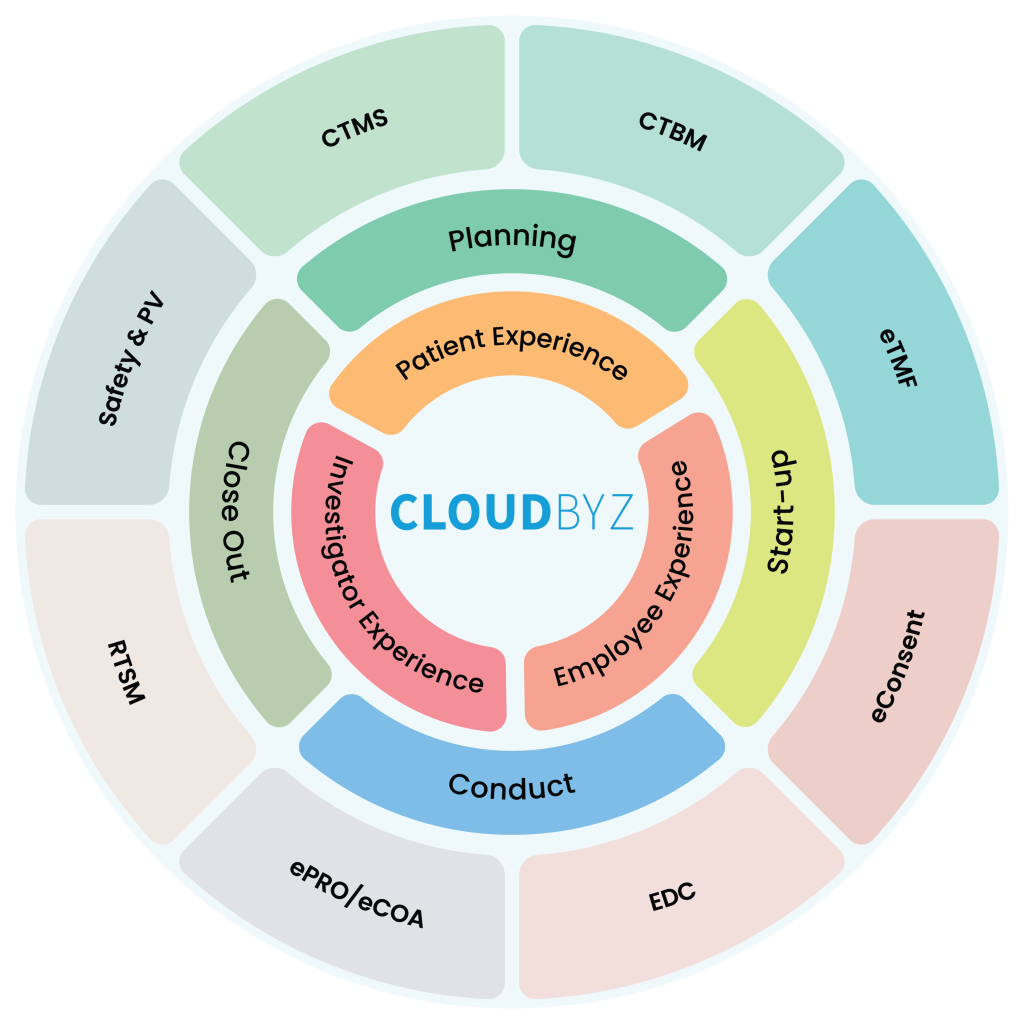
Here are some of the ways in which midsize CROs can use a unified clinical trial management platform to deliver services and achieve a competitive advantage:
In conclusion, midsize CROs can use a unified clinical trial management platform to deliver services and achieve a competitive advantage. By streamlining operations, improving data management, enhancing collaboration, managing patients better, improving reporting, and reducing costs, CROs can provide high-quality services that meet the needs of sponsors and investigators and differentiate themselves from their competitors.
Cloudbyz Unified Clinical Trial Management (CTMS) is a comprehensive and integrated solution designed to streamline clinical trial operations. Built on the Salesforce cloud platform, our CTMS provides real-time visibility and analytics across study planning, budgeting, start-up, study management, and close-out. With features like automated workflows, centralized data management, and seamless collaboration, Cloudbyz CTMS can help you achieve greater efficiency, compliance, and quality in your clinical operations. Contact us today to learn more about how Cloudbyz CTMS can help your organization optimize its clinical trial management processes.
To know more about the Cloudbyz Unified Clinical Trial Management Solution contact info@cloudbyz.com
Subscribe to our Newsletter

ISO 9001:2015 and ISO 27001:2013 Certified