Request a demo specialized to your need.
Subscribe to our Newsletter

Dinesh

Cloudbyz Unified Clinical Trial Management Solution (CTMS) is a comprehensive cloud-based platform that streamlines the management of clinical trials.
Cloudbyz Unified Clinical Trial Management Solution (UCTMS) is a cloud-based platform that can help academic research institutes manage their clinical trials more efficiently. UCTMS provides a centralized platform for managing various aspects of the clinical trial process, including patient recruitment, scheduling, data collection, and analysis. The platform enables organizations to streamline their clinical trial management processes, automate many of the manual tasks, and improve collaboration between stakeholders involved in the trial. UCTMS also helps organizations ensure compliance with regulatory guidelines and provides robust data security features. By providing real-time data access and analytics, UCTMS helps organizations to make informed decisions and optimize their clinical trial processes for better outcomes. Overall, UCTMS is an essential tool for organizations looking to conduct clinical trials more efficiently and effectively.
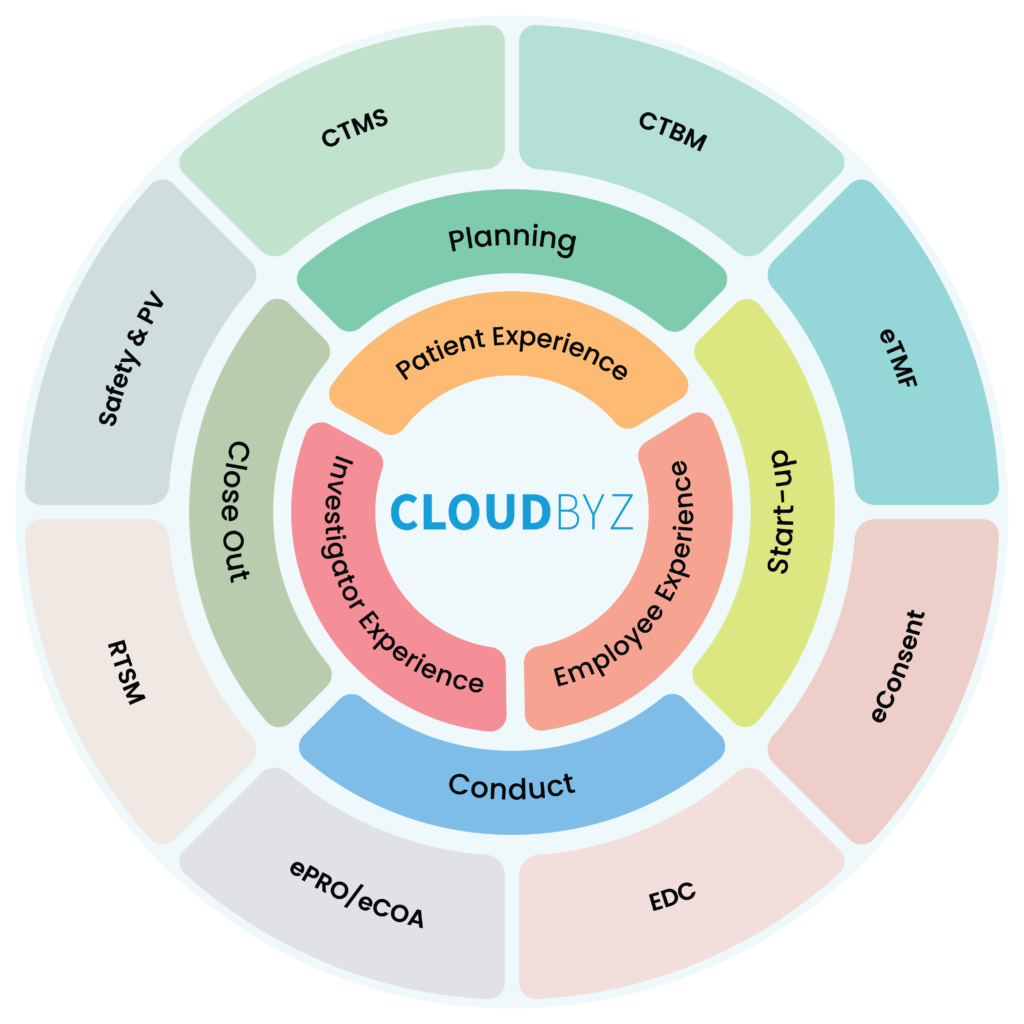
Cloudbyz Unified Clinical Trial Management Solution offers a wide range of benefits to the life sciences industry, including:
In summary, Cloudbyz Unified Clinical Trial Management Solution offers a comprehensive platform for managing all aspects of a clinical trial, from study design to closeout. It improves efficiency, productivity, data management, compliance, patient experience, finance management, and scalability. By using Cloudbyz CTMS, life sciences companies can accelerate the trial process, reduce costs, and improve patient outcomes.
Cloudbyz Unified Clinical Trial Management (CTMS) is a comprehensive, integrated solution to streamline clinical trial operations. Built on the Salesforce cloud platform, our CTMS provides real-time visibility and analytics across study planning, budgeting, start-up, study management, and close-out. Cloudbyz CTMS can help you achieve greater efficiency, compliance, and quality in your clinical operations with features like automated workflows, centralized data management, and seamless collaboration. Contact us today to learn how Cloudbyz CTMS can help your organization optimize its clinical trial management processes.
To know more about the Cloudbyz Unified Clinical Trial Management Solution contact info@cloudbyz.com
Subscribe to our Newsletter

ISO 9001:2015 and ISO 27001:2013 Certified