Request a demo specialized to your need.

Academic research institutes play a critical role in advancing scientific knowledge and developing new treatments for diseases. However, managing clinical trials is a complex and challenging task, especially for research institutes that are already stretched thin.
Unified Clinical Trial Management Solution Overview
Cloudbyz Unified Clinical Trial Management Solution (UCTMS) is a cloud-based platform that can help academic research institutes manage their clinical trials more efficiently. UCTMS provides a centralized platform for managing various aspects of the clinical trial process, including patient recruitment, scheduling, data collection, and analysis. The platform enables organizations to streamline their clinical trial management processes, automate many of the manual tasks, and improve collaboration between stakeholders involved in the trial. UCTMS also helps organizations ensure compliance with regulatory guidelines and provides robust data security features. By providing real-time data access and analytics, UCTMS helps organizations to make informed decisions and optimize their clinical trial processes for better outcomes. Overall, UCTMS is an essential tool for organizations looking to conduct clinical trials more efficiently and effectively.
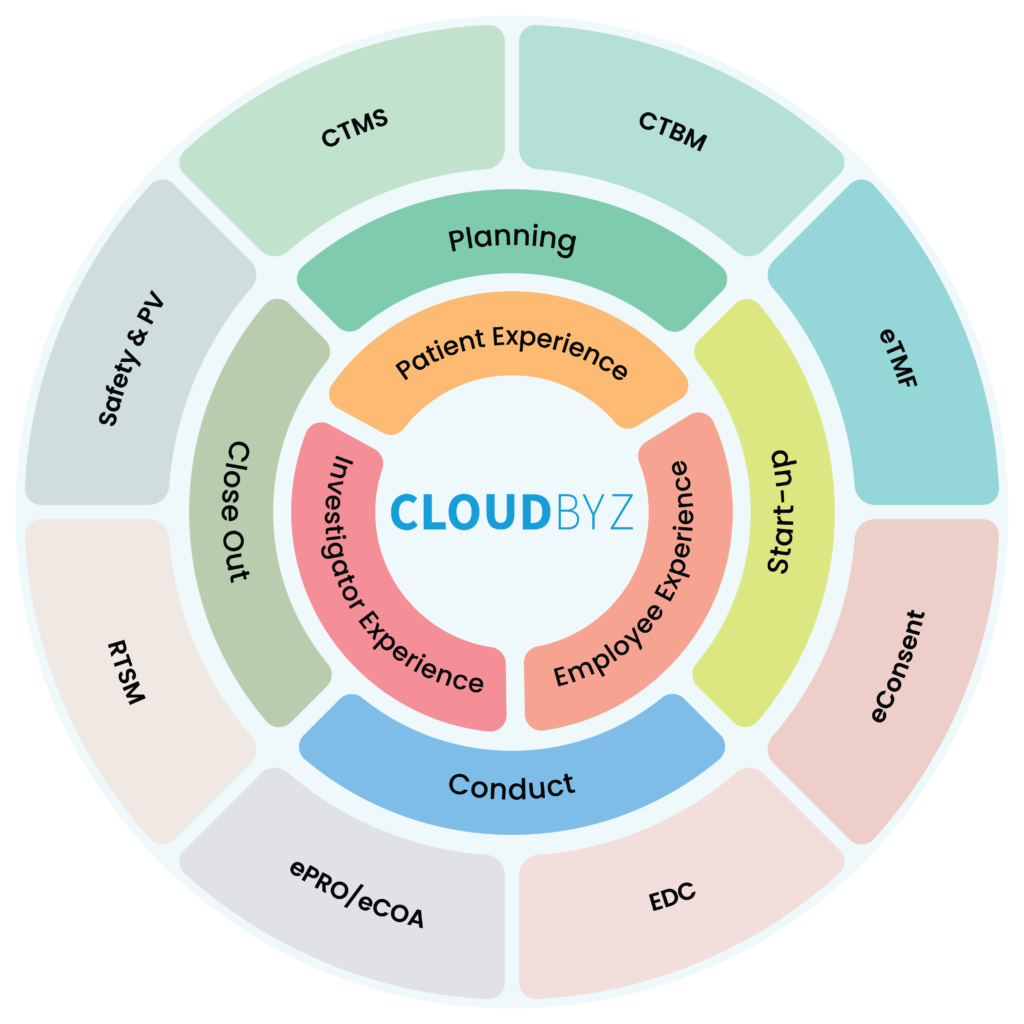
In this blog, we will discuss how Cloudbyz UCTMS can help unlock academic research institutes’ true potential.
- Streamlined Clinical Trial Management
Managing a clinical trial involves numerous tasks, including patient recruitment, scheduling, data collection, and analysis. Cloudbyz UCTMS provides a centralized platform for managing all these tasks, enabling research institutes to streamline their clinical trial management processes. With Cloudbyz UCTMS, research institutes can easily track the progress of their clinical trials, access real-time data, and collaborate with their research teams.
- Improved Collaboration
Collaboration is essential for the success of clinical trials, especially when multiple departments and stakeholders are involved. Cloudbyz UCTMS provides a centralized platform for collaboration, enabling research teams to share data, insights, and progress reports in real-time. This helps research institutes to avoid communication gaps, reduce errors, and enhance the overall efficiency of the clinical trial process.
- Increased Efficiency and Productivity
Cloudbyz UCTMS automates many of the manual tasks associated with clinical trial management, such as data entry, scheduling, and reporting. This automation saves research institutes time and resources, enabling them to focus on more critical tasks, such as data analysis and research. Cloudbyz UCTMS also provides analytics and reporting tools that enable research institutes to track their clinical trial performance and identify areas for improvement.
- Better Compliance and Regulatory Adherence
Compliance and regulatory adherence are critical to the success of any clinical trial. Cloudbyz UCTMS helps research institutes ensure that their trials comply with all applicable regulations and guidelines. The platform provides built-in compliance checks and audit trails, ensuring that all data is secure and accessible only to authorized personnel.
- Enhanced Data Management
Managing clinical trial data is a complex and challenging task, especially when dealing with large volumes of data. Cloudbyz UCTMS provides tools for managing data more efficiently, including data entry, data cleansing, and data analysis. The platform also provides tools for data visualization, enabling research teams to make sense of complex data sets more easily.
- Improved Patient Engagement
Patient engagement is critical to the success of any clinical trial. Cloudbyz UCTMS provides a patient portal that enables patients to stay informed about the trial’s progress and communicate with their healthcare providers. The portal also allows patients to schedule appointments, view their medical records, and access educational resources related to the trial.
- Increased Funding Opportunities
Cloudbyz UCTMS can help research institutes increase their funding opportunities by enabling them to conduct more clinical trials. The platform’s streamlined processes and automation tools enable research institutes to manage more trials efficiently, reducing the time and resources required for each trial. Additionally, Cloudbyz UCTMS provides billing and invoicing tools, enabling research institutes to bill sponsors and manage payments easily.
- Better Resource Management
Clinical trials require significant resources, including staff, equipment, and supplies. Cloudbyz UCTMS helps research institutes manage their resources efficiently, ensuring that they have the necessary resources available at the right time. The platform provides tools for scheduling staff, managing inventory, and tracking equipment usage. This helps research institutes to avoid overbooking, underutilization, and wastage of resources.
- Improved Data Security
Clinical trial data is sensitive and confidential, and it’s crucial to keep it secure. Cloudbyz UCTMS provides robust data security features, including encryption, authentication, and access control. This helps research institutes to protect their data from unauthorized access, breaches, and cyber-attacks. The platform also provides backup and disaster recovery features, ensuring that research institutes can recover their data in case of any data loss.
- Scalability and Flexibility
Cloudbyz UCTMS is a cloud-based solution that is scalable and flexible. It can adapt to the changing needs of research institutes, enabling them to add or remove users, change workflows, and customize reports. This flexibility helps research institutes to optimize their clinical trial management processes and improve their overall performance.
- Enhanced Quality Control
Quality control is essential for the success of clinical trials. Cloudbyz UCTMS provides tools for ensuring quality control, including automated data checks, discrepancy management, and review workflows. This helps research teams to identify and correct errors quickly, ensuring that the data is accurate and reliable.
- Increased Visibility
Cloudbyz UCTMS provides real-time visibility into clinical trial data, enabling research institutes to make informed decisions about their trials. The platform provides dashboards and reports that enable research teams to monitor the progress of their trials, identify trends, and track key performance indicators. This helps research institutes to optimize their trial management processes and improve their overall performance.
In conclusion, Cloudbyz Unified Clinical Trial Management Solution is a powerful tool that can help academic research institutes unlock their true potential. By providing streamlined processes, improved collaboration, enhanced patient engagement, better resource management, increased data security, scalability, and flexibility, Cloudbyz UCTMS can help research institutes conduct more clinical trials efficiently, generate more funding opportunities, and advance scientific knowledge. With Cloudbyz UCTMS, research institutes can focus on what they do best – advancing scientific knowledge and developing new treatments for diseases.
Cloudbyz Unified Clinical Trial Management (CTMS) is a comprehensive and integrated solution designed to streamline clinical trial operations. Built on the Salesforce cloud platform, our CTMS provides real-time visibility and analytics across study planning, budgeting, start-up, study management, and close-out. With features like automated workflows, centralized data management, and seamless collaboration, Cloudbyz CTMS can help you achieve greater efficiency, compliance, and quality in your clinical operations. Contact us today to learn more about how Cloudbyz CTMS can help your organization optimize its clinical trial management processes.
To know more about Cloudbyz Unified Clinical Trial Management Solution contact info@cloudbyz.com
Subscribe to our Newsletter

