Request a demo specialized to your need.
Pharmacovigilance is a continuous process of collection and analysis of data relating to the detection, assessment, understanding, and prevention of adverse events of medicines and medical devices. Pharmacovigilance is critical for the Healthcare industry for approval of new drugs and devices and to further promote their safe use post-approval. The Challenges to Pharmacovigilance were discussed in our previous blog and in this article, we propose a few automation solutions to address those.
The increased demand for drug safety by regulatory authorities is leading multinational pharmaceutical companies to Pharmacovigilance Outsourcing (PVO). Vendors offer setting up an in-house Pharmacovigilance solution, with the cost efficiencies and high accuracy leading to effective Pharmacovigilance process.
Standard Medical Coding
The adverse event is classified using medical terms in MedDRA (Medical Dictionary for Regulatory activities) and it is updated twice a year. This helps all concerned authorities to readily exchange and analyze the data in a standard manner. The accurately coded data is exportable and it can be utilized to review product performance, as well as aggregate and report to the regulatory authority.
MedWatch Information in Cloud-based solution
MedWatch is FDA’s reporting system for adverse events. It is a spontaneous reporting system that allows the consumer or HCP or the patient to report adverse events voluntarily. And some solutions like Cloudbyz Safety solution enable to enter additional details to the MedWatch 3500A form. This information is used for generating regulatory and MedWatch reports.
Adverse event management
Adverse events reported are classified as AE, SAE and so on in the Solution. The seriousness criteria of each event and the outcome of exposure are all encoded. Not all cases are to be reported to the regulatory authorities. The system captures all the events and validates the existing and new SAE and helps in periodic PSUR (Periodic safety update report)reporting as it reduces the time and increases efficiency.
Safety Solution enables to report data based on regulatory requirements, configured to look at the Seriousness, causality, and outcome.
Solutions to Data mining for adverse events
Machine learning techniques are used to mine Medical literature as well as large data sets to identify proactively the adverse event signals and the extent of the impact. Algorithms are trained with large data sets and well-annotated content to produce the best results. NLP (Natural Language Processing) algorithm is used to mine published medical literature by automatically extracting relevant information from the database.
EHR(Electronic health records) databases are the most proactive method for Pharmacovigilance as it contains real-time data and is usually created and maintained by HCP’s. For example, A large group of patients administered with the same drug for the same disease can be identified and analyzed for the extent of exposure in case of adverse events.
EHR database is used for validation of adverse event signals that are once initiated from the spontaneous reports.
Integrated Cloud-based Solution
Cloud-based solutions provide 360-degree visibility and efficiency in every step. The solution enables pharma, biotech, and medical devices companies to make faster and better safety decisions, optimize global compliance, and easily integrate risk management. Adverse events are directly reported by the patient or the Healthcare Provider or via phone, email or the web, all these are recorded in the Cloud-based Safety solutions from any device, anywhere and any time for automated assessment and reporting. All the adverse events reported either during the clinical trial or during the post-marketing phase are recorded separately and analyzed. The nature of the event, serious criteria, drug name, dosage and type and length of exposure are noted.
All the above mentioned improved methods, tools are in the early stages of development and are sure to make a huge impact in the way Pharmacovigilance is performed in the future. With all these technological advancement PV staff can detect and solve signals and generate reports on the safety of the medicines and products. Such a Standardized system is flexible, agile and reduces costs and risks in the Pharmacovigilance process.
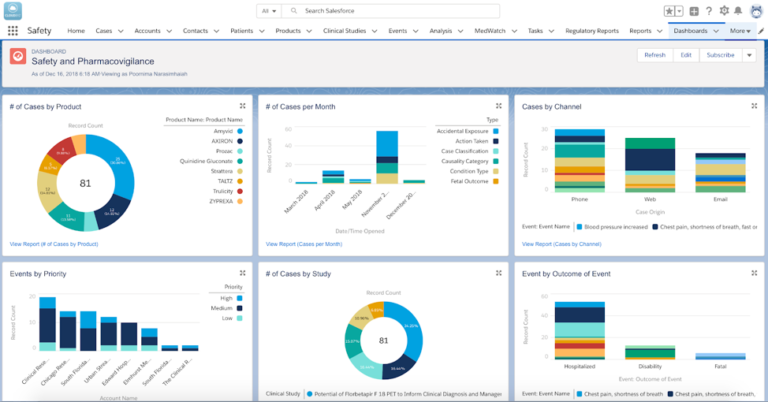
Cloudbyz Safety & Pharmacovigilance solution accelerator built 100% native on Salesforce Service Cloud offers a 360-degree view across R&D and commercial and helps enable pharma, biotech, and medical devices companies to make faster and better safety decisions, optimize global compliance, and easily integrate risk management.
References
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5969211/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3108683/
http://www.cloudbyz.com/Cloudbyz_safety.html
By Mythri Raghunandan
Subscribe to our Newsletter

