Request a demo specialized to your need.
Overview
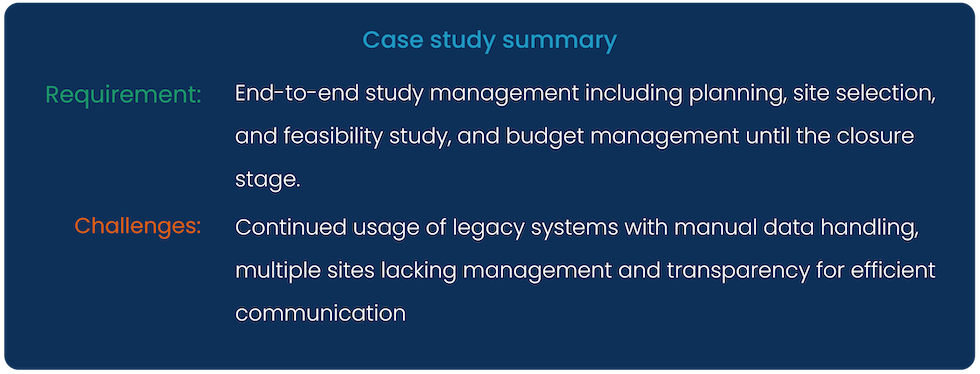
Cloudbyz was approached by a US-based global Fortune 500, a wholesale drug distribution company with a business requirement for end-to-end study management. Being a large company, our client (a CRO) struggled to effectively manage several clinical trial sites and collaborate (in terms of data visibility, approvals, and performance mapping) efficiently with all the stakeholders involved.
Company Information
Our client is an American drug wholesale company with over 4 decades of existence in the industry. In particular, they offer drug distribution and consulting services related to medical business operations and patient services. The company’s operational vastness can be estimated by its continuously growing employee base of ~22,000 (as of 2019) and a top-line earning of US$ 18,000cr!
Constraints Faced by the Client.
We at Cloudbyz, to gain a better perspective, analyzed our client’s existing process to realize the following constraints:
- Continued use of excel-driven format for entire budgeting and contracting process
- Tedious and manual handling and tracking of invoices and payments
- Lack of collaboration between the sponsors and sites due to no transparent information flow
Our client was quite vocal about their requirements. They stressed eradicating their legacy process (wherever possible) and replacing it with software solutions pertaining to the rapidly digitalized ecosystem and changing clinical trial landscape.
The requisite included:
- Improved collaboration between stakeholders with the transparent flow of data, milestone, and study-related information
- Expedite the site identification process to enable a faster trial process and evade any opportunity loss
- Ensure a completely rational and data-driven site selection to match study site criteria
Solutions Delivered by Cloudbyz
After a thorough investigation of the requirements and scope of work, cloudbyz analyzed that our client needed an in and out streamlined clinical trial management solution and therefore we implemented our Clinical Trial Site and Budget Management Solution via Cloudbyz CTMS.
Our approach was strategic and we planned to move in a systematic manner. The first stage included designing, deployment, and implementation of a comprehensive budget management solution. We also designed site visit plans based on specified clinical studies as part of our CTMS solution offering.
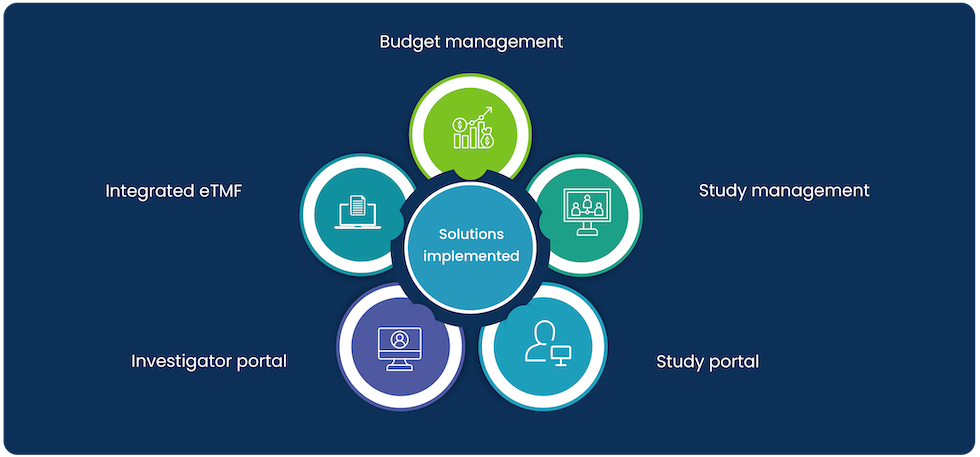
This was followed by an end-to-end study management solution including planning, start-up, conduct, and close-out.
Cloudbyz delivered multiple solution capabilities which included:
- Track and manage site details (including former database) for eased site identification
- Easy to track and analyze digitalized site performance metrics
- Online site feasibility study and site verification process
- Availability of centralized regulatory document checklist
- Detailed, real-time overview and management of site and study budgets
- Management of MSA, standard task, visit, and procedure-based rate card for sites and sponsors
- Site management and monitoring
- Subject enrollment management
- Management and reconciliation for payments, invoices, and receipts
For a holistic solution delivery, Cloudbyz also implemented our integrated eTMF, investigator portal, and sponsor portal for our client
Intrigued by our service capability? We would love to give a product walkthrough.
The Final Results
The establishment of a streamlined systematic process through Cloudbyz products fulfilled every requisite on our client’s checklist. We succeeded in delivering a digitalized, centralized and cloud-based CTMS solution for improved efficiency for the entire study management process.
The company benefited from an easy-to-access, multi-gadget configurable SaaS solution for better collaboration, communication, and trial execution. Most importantly, we provided our client – a CRO, with improved and ‘one-stop view for all the study and site related data, process flow, and process management.
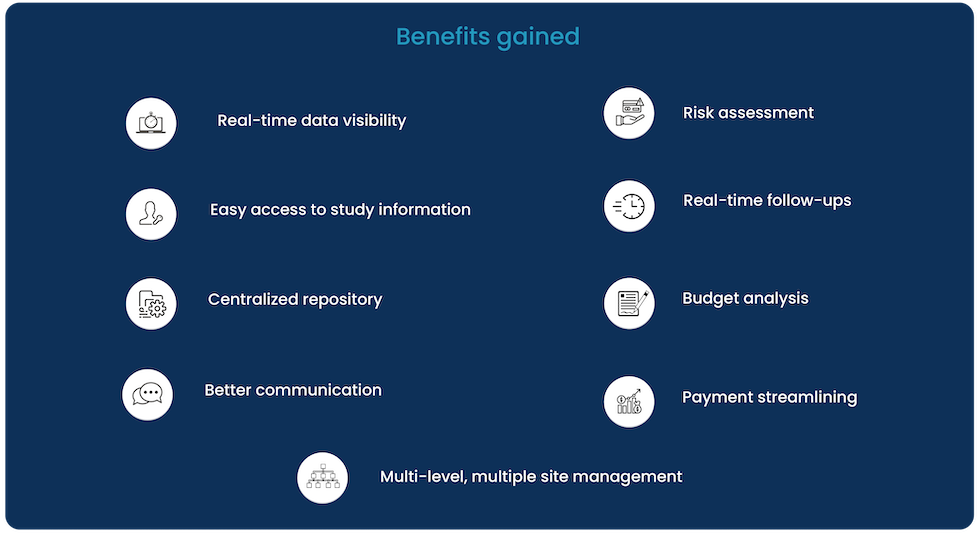
The major benefits derived from Cloudbyz’s service delivery included:
- Proper assessment of budgets facilitated by real-time comparative visibility for planned budget v/s actual cost
- Time and cost efficiency gained through quicker site assessment, selection and start-up with real-time centralized view of all study listing, eligibility, and survey data
- Supported faster site start-up with real-time effective collaboration on CDA, budgets, and contracts
- Improved and regulated site inspection and operational process with document listing and regulatory adherence management
- Multi-level site collaboration and study monitoring with geographically dispersed sites via investigator portal
- Real-time analysis of adverse events and protocol deviations with on-spot follow-ups to evade complexities
- Complete tracking and management efficiency of subject enrollment for study sites.
As a result, the entire study process gained consistency along with the digitalized ecosystem.
About Cloudbyz:
Cloudbyz is a cloud-based, salesforce-built clinical trial solution provider to the life science industry delivering intuitive, flexible, and scalable products with rapid deployment functionality.
Subscribe to our Newsletter

