Request a demo specialized to your need.
Subscribe to our Newsletter

Dinesh
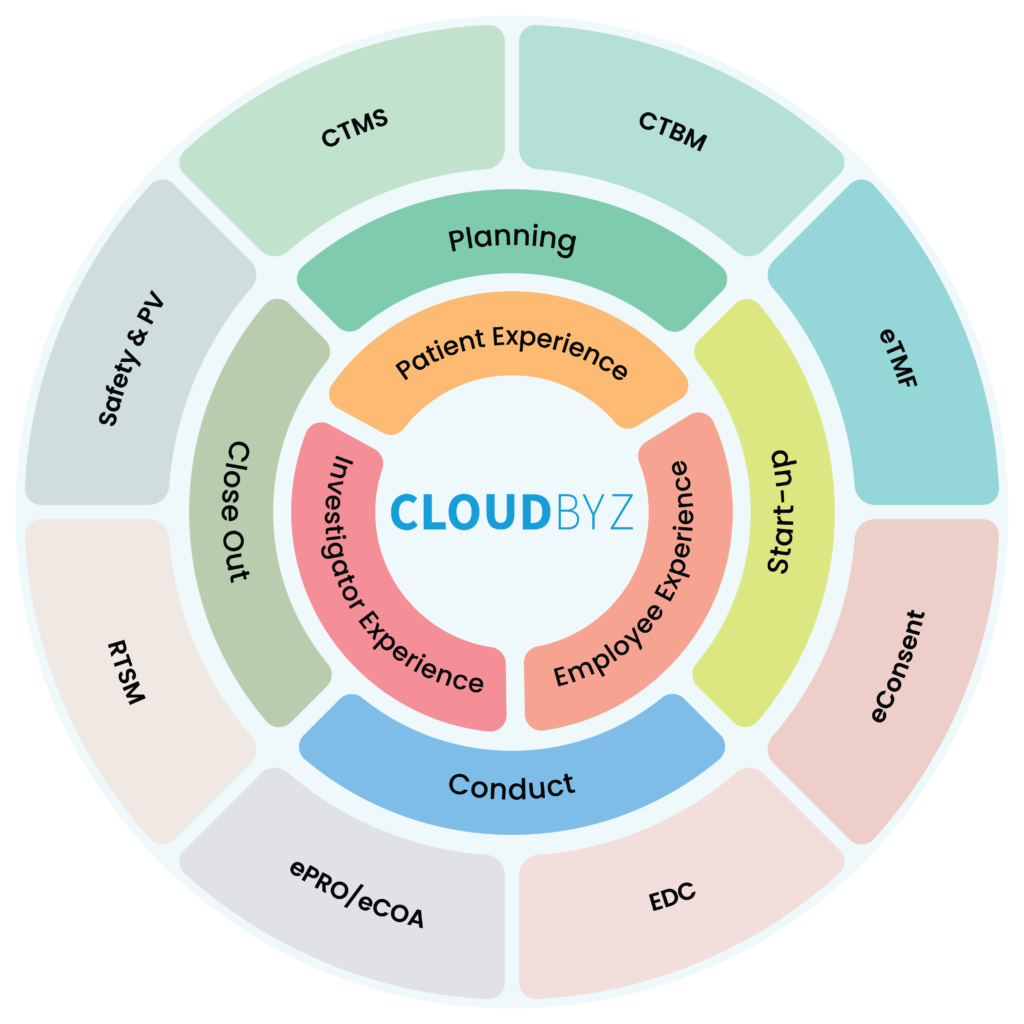
Cloudbyz specializes in providing cloud-based solutions for the healthcare industry. One of its flagship offerings is its eClinical Cloud, built on the Salesforce platform. This solution is designed to meet the unique needs of clinical research organizations (CROs) and pharmaceutical companies, offering a range of features to help them streamline their operations, manage clinical trials, and ensure compliance with regulatory requirements.
One of the key advantages of Cloudbyz’s eClinical Cloud is that it is built on the Salesforce platform. Salesforce is a highly popular and widely used cloud platform that is known for its flexibility, scalability, and ease of use. By leveraging Salesforce’s platform capabilities, Cloudbyz is able to offer a highly customizable and configurable solution that can be tailored to meet the specific needs of each client.
The eClinical Cloud is also highly secure and compliant with regulatory requirements, which is critical for companies operating in the highly regulated healthcare industry. The solution includes a range of features to help CROs and pharmaceutical companies comply with regulatory requirements, including electronic signatures, audit trails, and document versioning.
But what sets Cloudbyz apart from its competitors is its focus on delivering a comprehensive solution that covers the entire clinical trial lifecycle. The eClinical Cloud includes modules for study design, subject enrollment, randomization and dosing, data capture and management, monitoring, and reporting. By offering a comprehensive solution that covers all aspects of the clinical trial process, Cloudbyz is able to help its clients save time and money, while also improving the quality of their data.
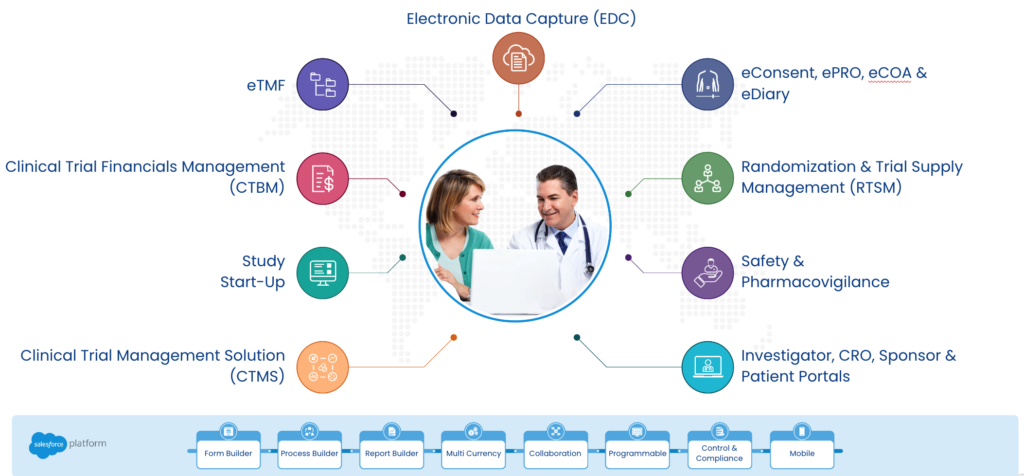
In addition, Cloudbyz’s eClinical Cloud is highly scalable, which means it can grow and adapt to meet the changing needs of its clients. As clinical trials become more complex and data-intensive, the eClinical Cloud can scale to handle the increased volume of data and support the integration of new technologies such as wearables and remote monitoring.
The appeal of industry clouds, as described in a Deloitte article, is that industry-specific functionality and expertise that is tailored to the unique needs of each industry. Cloudbyz’s eClinical Cloud is a prime example of this trend, offering a comprehensive solution that is specifically designed for the healthcare industry.
The article also notes that industry clouds are becoming increasingly popular due to their ability to address industry-specific challenges and pain points, such as regulatory compliance, data security, and interoperability. Cloudbyz’s eClinical Cloud addresses all of these challenges and more, making it an attractive option for Biotech, Medical Devices, CROs and pharmaceutical companies looking to streamline their clinical trial operations.
In conclusion, Cloudbyz is leading the way with its eClinical Cloud built on Salesforce. By leveraging the power and flexibility of the Salesforce platform, Cloudbyz is able to offer a highly configurable and customizable solution that meets the unique needs of CROs and pharmaceutical companies. With its comprehensive range of features, focus on compliance and security, and ability to scale and adapt to changing needs, Cloudbyz’s eClinical Cloud is a prime example of the growing trend towards industry clouds in the healthcare industry.
Cloudbyz Unified Clinical Trial Management (CTMS) is a comprehensive, integrated solution to streamline clinical trial operations. Built on the Salesforce cloud platform, our CTMS provides real-time visibility and analytics across study planning, budgeting, start-up, study management, and close-out. Cloudbyz CTMS can help you achieve greater efficiency, compliance, and quality in your clinical operations with features like automated workflows, centralized data management, and seamless collaboration. Contact us today to learn how Cloudbyz CTMS can help your organization optimize its clinical trial management processes.
To know more about the Cloudbyz Unified Clinical Trial Management Solution contact info@cloudbyz.com
Subscribe to our Newsletter

ISO 9001:2015 and ISO 27001:2013 Certified