Request a demo specialized to your need.
Clinical trials are an essential part of the drug development process. They are designed to ensure that new drugs and treatments are safe, effective, and reliable before they are made available to the public. The success of a clinical trial depends on the efficient management of the study, which is the responsibility of the sponsor. In this blog post, we will discuss the roles and responsibilities of a sponsor in clinical trial management.
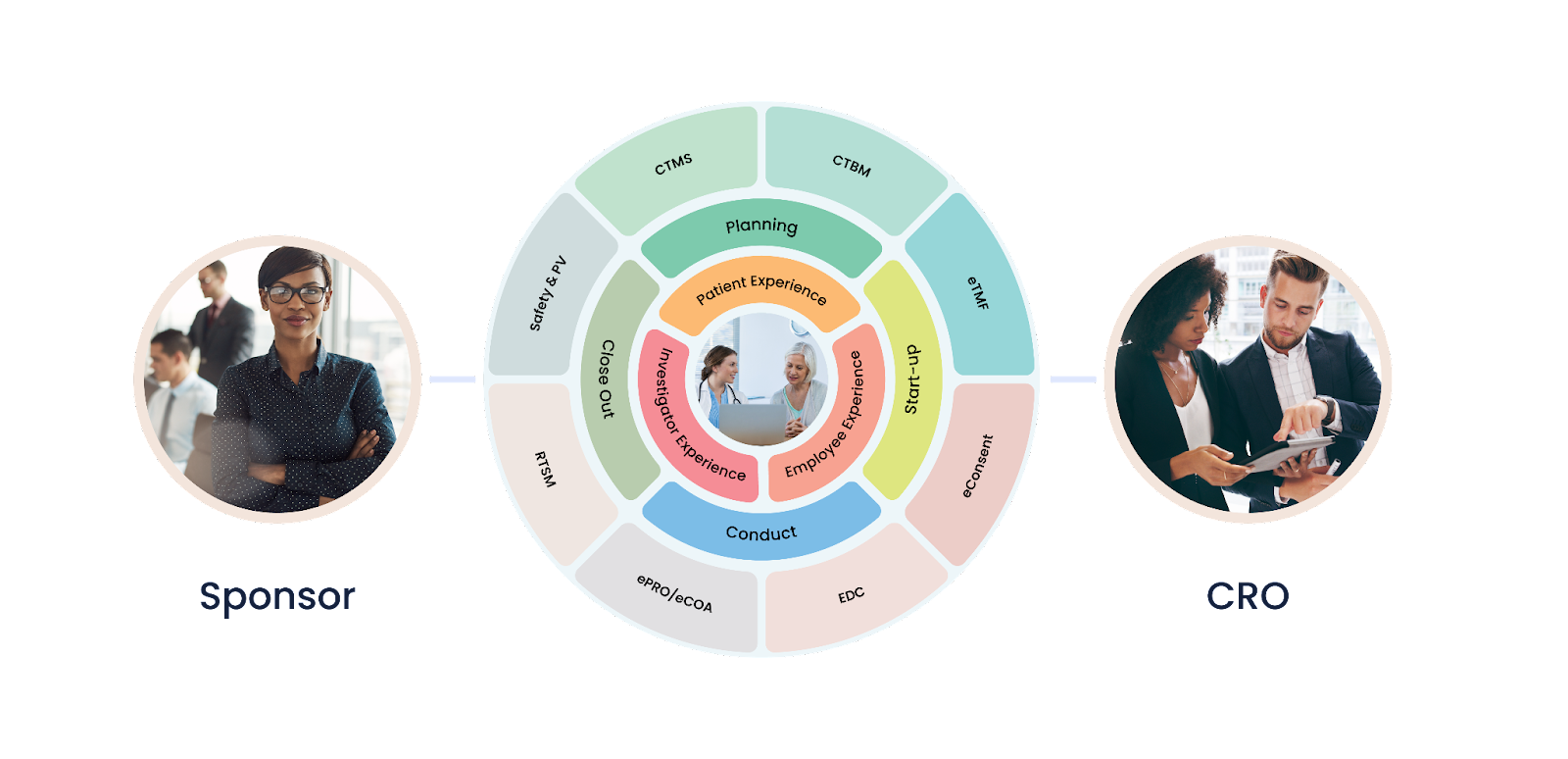
What is a Sponsor?
The sponsor is the organization or individual that takes responsibility for the initiation, management, and financing of a clinical trial. In most cases, the sponsor is a pharmaceutical company, biotech company, or academic institution. The sponsor is responsible for ensuring that the clinical trial is conducted in compliance with applicable regulatory requirements, ethical principles, and the protocol.
Roles and Responsibilities of a Sponsor in Clinical Trial Management
- Protocol Development
The sponsor is responsible for developing the clinical trial protocol, which outlines the objectives, design, methodology, and statistical analysis plan for the trial. The protocol is a critical document that guides the conduct of the trial and ensures that the trial is conducted in a standardized manner.
- Regulatory Compliance
The sponsor is responsible for obtaining regulatory approval for the trial and ensuring that the trial complies with all applicable regulations and guidelines. The sponsor must submit an Investigational New Drug (IND) application to the appropriate regulatory agency before conducting the trial. The sponsor is also responsible for obtaining approval from the Institutional Review Board (IRB) or Ethics Committee (EC) before the trial begins.
- Funding and Budget Management
The sponsor is responsible for providing funding for the trial and managing the trial budget. The sponsor must ensure that there is adequate funding to cover the costs of conducting the trial, including the costs of study drug, study supplies, monitoring, and data management.
- Monitoring and Oversight
The sponsor is responsible for monitoring the progress of the trial, ensuring that it is conducted according to the protocol, and overseeing the activities of the CRO and clinical site. The sponsor may conduct on-site visits to the clinical site to monitor the conduct of the trial and ensure that the trial is conducted according to the protocol.
- Safety Reporting
The sponsor is responsible for ensuring that adverse events and other safety issues are reported to the appropriate regulatory authorities and that appropriate measures are taken to mitigate any risks to trial participants. The sponsor must have a system in place for collecting, reviewing, and reporting adverse events and other safety information.
- Data Management
The sponsor is responsible for managing the collection, storage, and analysis of trial data. The sponsor must ensure that the data is collected and recorded accurately and that it is securely stored. The sponsor is also responsible for ensuring that the data is analyzed according to the statistical analysis plan outlined in the protocol.
The sponsor plays a critical role in the successful management of a clinical trial. The sponsor is responsible for ensuring that the trial is conducted in compliance with all applicable regulations and guidelines, that adequate funding is available to conduct the trial, and that the trial is conducted according to the protocol. The sponsor must also ensure that adverse events and other safety issues are reported and that the data is collected, stored, and analyzed accurately. By fulfilling these roles and responsibilities, the sponsor can help to ensure the success of the trial and the development of new, effective treatments for patients.
Contract Research Organization (CRO)
Contract Research Organizations (CROs) play a vital role in clinical trial management. They are specialized companies that provide support to sponsors in conducting clinical trials. Their role is to ensure that the clinical trial is conducted efficiently and effectively, while complying with regulatory requirements and ethical principles. In this blog post, we will discuss the roles and responsibilities of CROs in clinical trial management.
What is a CRO?
A CRO is a company that provides support to sponsors in the conduct of a clinical trial. The CRO may be contracted by the sponsor to manage various aspects of the clinical trial, such as site selection, participant recruitment, data management, monitoring, and safety reporting. CROs play an essential role in ensuring that clinical trials are conducted efficiently, effectively, and in compliance with regulatory requirements and ethical principles.
Roles and Responsibilities of CROs in Clinical Trial Management
- Site Selection and Management
The CRO is responsible for identifying and selecting suitable clinical sites for the trial. The CRO must ensure that the selected sites have the necessary infrastructure and expertise to conduct the trial. The CRO is also responsible for managing the activities of the clinical sites, including training, monitoring, and support.
- Study Design and Protocol Development
The CRO may provide input into the design of the trial and assist with the development of the protocol. The CRO must ensure that the trial is designed in a way that is feasible, ethical, and scientifically sound. The CRO must also ensure that the protocol is followed during the conduct of the trial.
- Regulatory Compliance
The CRO is responsible for ensuring that the trial complies with all applicable regulatory requirements and guidelines. The CRO must ensure that the trial is conducted in compliance with the International Conference on Harmonization (ICH) guidelines, Good Clinical Practice (GCP) guidelines, and local regulatory requirements.
- Data Management
The CRO is responsible for managing the collection, storage, and analysis of trial data. The CRO must ensure that the data is collected and recorded accurately and that it is securely stored. The CRO is also responsible for ensuring that the data is analyzed according to the statistical analysis plan outlined in the protocol.
- Monitoring and Oversight
The CRO is responsible for monitoring the progress of the trial and ensuring that it is conducted according to the protocol. The CRO must ensure that the clinical sites are following the protocol and that the trial is being conducted in compliance with regulatory requirements and ethical principles.
- Safety Reporting
The CRO is responsible for ensuring that adverse events and other safety issues are reported to the appropriate regulatory authorities and that appropriate measures are taken to mitigate any risks to trial participants. The CRO must have a system in place for collecting, reviewing, and reporting adverse events and other safety information.
CROs play a vital role in clinical trial management. They provide support to sponsors in conducting clinical trials efficiently and effectively, while ensuring that the trial is conducted in compliance with regulatory requirements and ethical principles. The CRO’s roles and responsibilities include site selection and management, study design and protocol development, regulatory compliance, data management, monitoring and oversight, and safety reporting. By fulfilling these roles and responsibilities, the CRO can help to ensure the success of the trial and the development of new, effective treatments for patients.
Clinical Sites
Clinical sites play a crucial role in the success of clinical trials. They are responsible for conducting the trial according to the protocol and ensuring that the data collected is accurate and reliable. Clinical site personnel are in direct contact with study participants and are responsible for ensuring that they receive appropriate care and that their rights and welfare are protected. In this blog post, we will discuss the roles and responsibilities of clinical sites in clinical trial management.
What is a Clinical Site?
A clinical site is a facility, such as a hospital or clinic, where clinical trials are conducted. Clinical sites are responsible for conducting the trial according to the protocol and ensuring that the data collected is accurate and reliable. Clinical sites are responsible for recruiting and enrolling study participants, administering the study intervention, and collecting data.
Roles and Responsibilities of Clinical Sites in Clinical Trial Management
- Participant Recruitment
The clinical site is responsible for identifying and recruiting suitable participants for the trial. The site must ensure that participants meet the eligibility criteria for the trial and that they understand the study procedures and risks.
- Informed Consent
The clinical site is responsible for obtaining informed consent from trial participants before they are enrolled in the trial. The site must ensure that the participant understands the study procedures, the risks and benefits of participation, and that they have the right to withdraw from the trial at any time.
- Study Conduct
The clinical site is responsible for conducting the trial according to the protocol. The site must ensure that the study procedures are followed, the study intervention is administered correctly, and the data is collected accurately.
- Safety Reporting
The clinical site is responsible for reporting adverse events and other safety issues to the sponsor and regulatory authorities. The site must have a system in place for collecting, reviewing, and reporting adverse events and other safety information.
- Compliance with Regulations and Guidelines
The clinical site is responsible for complying with all applicable regulations and guidelines. The site must ensure that the trial is conducted in compliance with the International Conference on Harmonization (ICH) guidelines, Good Clinical Practice (GCP) guidelines, and local regulatory requirements.
Clinical sites play a crucial role in clinical trial management. They are responsible for recruiting and enrolling study participants, administering the study intervention, and collecting data. The clinical site’s roles and responsibilities include participant recruitment, obtaining informed consent, study conduct, safety reporting, and compliance with regulations and guidelines. By fulfilling these roles and responsibilities, clinical sites can help to ensure the success of the trial and the development of new, effective treatments for patients.
Clinical trial management is a complex process that requires collaboration between various stakeholders. Sponsors, CROs, and clinical sites all have important roles and responsibilities in ensuring that a clinical trial is conducted safely, ethically, and in compliance with all applicable regulations and guidelines. By working together and fulfilling their respective roles, these stakeholders can help to ensure the successful completion of a clinical trial and the development of new, effective treatments for patients.
Cloudbyz Unified Clinical Trial Management (CTMS) is a comprehensive, integrated solution to streamline clinical trial operations. Built on the Salesforce cloud platform, our CTMS provides real-time visibility and analytics across study planning, budgeting, start-up, study management, and close-out. Cloudbyz CTMS can help you achieve greater efficiency, compliance, and quality in your clinical operations with features like automated workflows, centralized data management, and seamless collaboration. Contact us today to learn how Cloudbyz CTMS can help your organization optimize its clinical trial management processes.
To know more about the Cloudbyz Unified Clinical Trial Management Solution contact info@cloudbyz.com
Subscribe to our Newsletter

