Customer Overview
The customer a leading multinational pharmaceutical company, is committed to providing affordable and innovative medicines for healthier lives. To scale its clinical trials operations, they turned to Cloudbyz for a comprehensive safety solution.
Challenges
The customer was struggling with several issues due to the use of manual, paper-based processes and disconnected legacy systems. These outdated methods led to inefficiencies, lack of collaboration, and increased costs, ultimately delaying their time-to-market. Additionally, working with multiple CROs without a centralized system forced them to rely on CRO-specific tools, creating information silos and preventing effective global trial management.
What it needed:
- Compliance with global regulatory requirements for safety and reporting.
- A centralized system to streamline operations and unify CRO data.
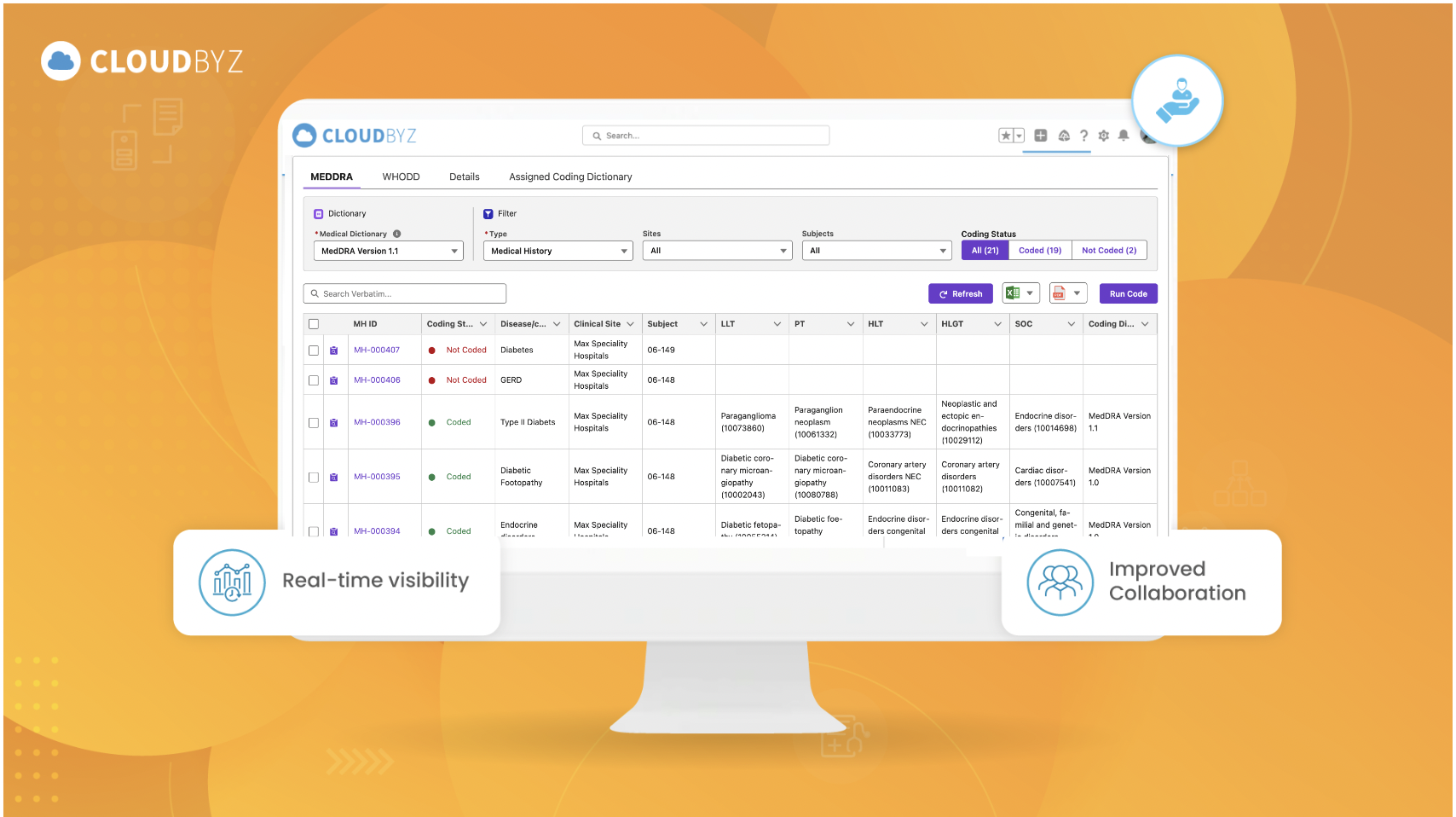
- End-to-end visibility across all clinical trial activities.
- Improved collaboration between internal teams and external partners.
- Real-time data and analytics to improve decision-making.
Solution
The customer chose Cloudbyz’s unified eClinical solutions, built on the Salesforce cloud platform, to streamline their operations. With Cloudbyz’s integrated clinical trial management system, it can now run end-to-end global trials and analyze safety data effectively. Integration with MedDRA and WHODrug coding dictionaries ensures they meet all regulatory safety reporting requirements.
Benefits
Cloudbyz delivered an integrated solution that offers real-time data visibility and analytics across the entire trial lifecycle—from study planning, budgeting, and startup, to data collection, study management, and closeout. By bringing together CRO data into a single system, the customer significantly improved the quality and accuracy of safety data across all their study sites. Some highlights are mentioned below:
- In a large post-marketing study, 85% of serious ICSRs were processed within 7 days, enhancing both compliance and patient safety outcomes.
- The customer achieved 95% compliance with US safety reporting timelines using the Cloudbyz Safety & PV Database, leading to faster and more reliable safety reporting.
- Audit preparation time was reduced by 40%, streamlining compliance processes and reducing operational overhead.
- Audit response timelines improved by 30%, allowing it to submit faster and more accurate reports during regulatory inspections.
- 90% of safety signals were identified and evaluated within 30 days, ensuring proactive risk management and enhanced participant safety.
Additionally, with Cloudbyz’s EDC solution, the customer can now quickly build studies using a point-and-click interface. This tool allows users to easily design forms and collect data, improving the overall efficiency of their trials.
With inspection-ready trial documents and real-time collaboration with study partners, the customer now complies with essential regulatory requirements, including FDA 21 CFR Part 11, GCP, GAMP5, HIPAA, and EU-GDPR.
