Customer Overview
The company was formed in 2020 by the merger of 2 reputed clinical research establishments in New Zealand, and is the country's leading early phase clinical research provider. The customer's aim is the development of research infrastructure with the promise of delivering better treatment options.
Challenges
The customer faced challenges due to the lack of a centralized trial management system for conducting clinical trials across multiple facilities in different locations. Recognizing the industry’s shift toward digitalization, it sought a unified trial solution to streamline operations and improve overall efficiency. To address these needs, the customer partnered with Cloudbyz to implement a comprehensive, integrated system that could support all aspects of trial management, including participant recruitment, data collection, and compliance.
Solution
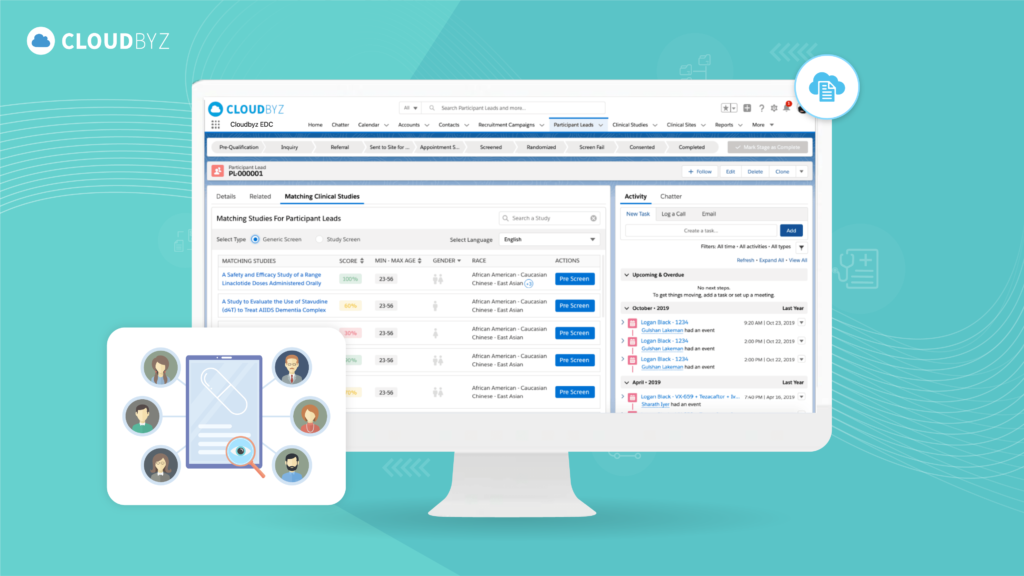
Cloudbyz's solutions—Participant Recruitment, eDOA, eConsent, and eSource—were adopted to enhance participant engagement and streamline trial operations. The Participant Recruitment solution improved the process of identifying and enrolling participants, while the eConsent solution provided a tailored electronic consent process, ensuring both compliance and an enhanced participant experience. The eDOA solution automated the delegation of authority process, simplifying site management and authorizations. In addition, the implementation of Cloudbyz eSource enabled direct electronic capture of source data, allowing the customer to eliminate paper-based processes and enhance data accuracy, integrity, and real-time access. This further streamlined data collection and reduced manual transcription errors, optimizing trial workflows across multiple sites.
Benefits
By replacing disconnected legacy systems with Cloudbyz’s highly integrated eClinical platform, the customer now benefits from a one-stop solution for all trial requirements, including participant registration, study enrollment, and real-time participantdata collection. The integration of the eSource solution added an extra layer of efficiency by automating data capture and management, which not only reduced operational burdens but also improved data quality and compliance with regulatory requirements. This centralized cloud-based platform has significantly streamlined operations, improving the overall efficiency and scalability of the customer’s clinical operations and enabling faster, more accurate decision-making throughout the trial lifecycle.
