Customer Overview
Established in 1993, the customer is one of Australia's largest and most experienced clinical trial establishments. Their team specializes in a range of early-phase clinical trials and and first-time-in-human studies. It has conducted more than 200 studies to date.
Challenges
The customer’s legacy technology infrastructure was heavily siloed, which is a common issue in clinical trials today, especially when scaling is required. The inability to scale quickly, along with fragmented communication channels between departments and external stakeholders, often results in inefficiencies, delayed decision-making, and increased operational costs. In clinical trials, such delays can severely impact patient recruitment, data collection, and overall trial timelines, ultimately affecting time-to-market for new therapies. Moreover, the maintenance of multiple disconnected systems escalates IT overhead, complicating trial management and compliance efforts.
Solution
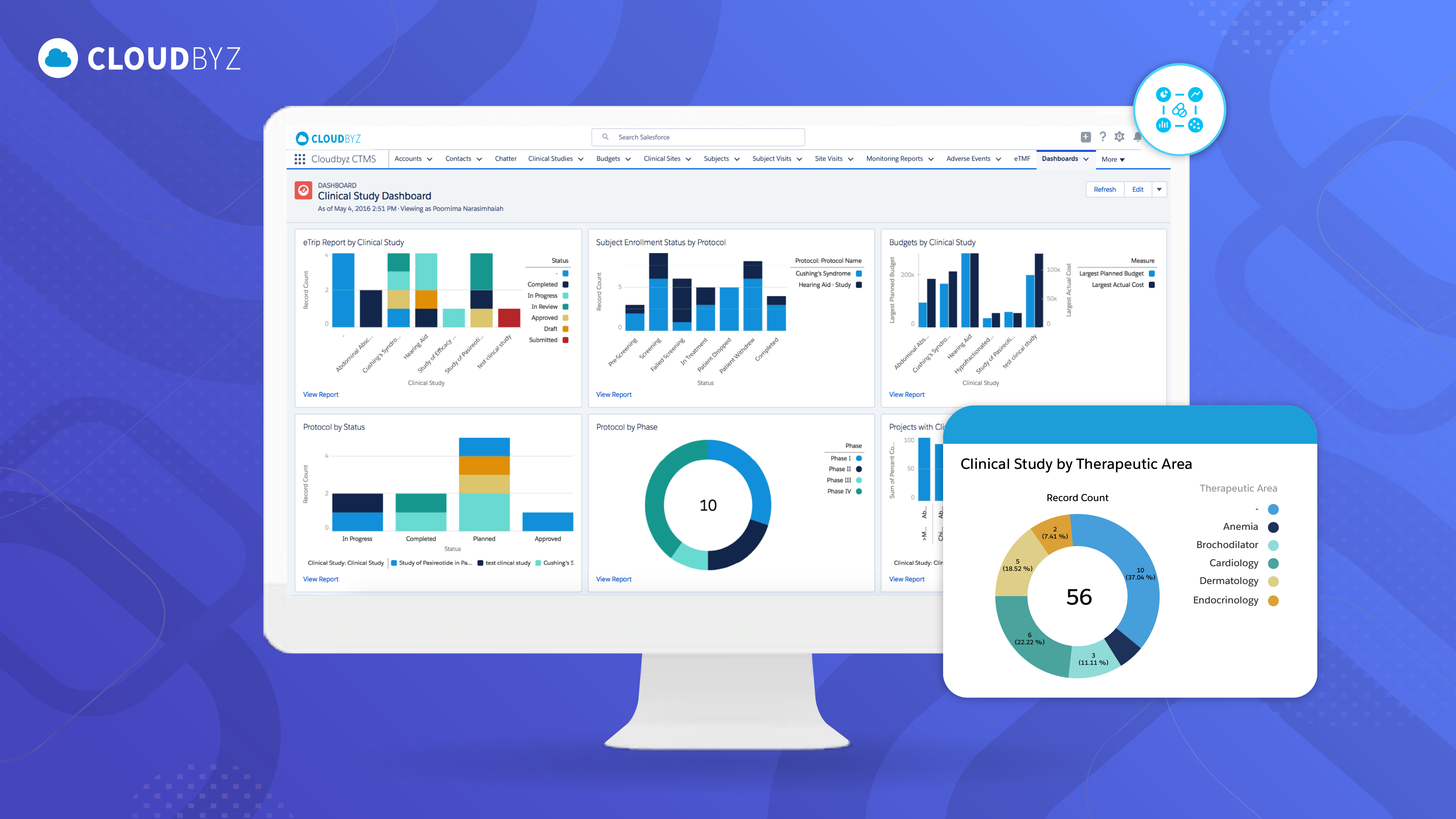
The customer adopted Cloudbyz’s unified eClinical solutions to address the inefficiencies caused by their legacy systems. These solutions included critical components such as Clinical Trial Management Systems (CTMS), Patient Recruitment, eConsent, and eRegulatory, all integrated into a single platform. By consolidating these functionalities, the customer was able to manage end-to-end trial processes more seamlessly, eliminating the need for multiple, disconnected systems. This integration ensured that all trial-related activities, from patient recruitment to regulatory compliance, were housed under one comprehensive system, significantly streamlining operations.
Benefits
By adopting Cloudbyz's unified eClinical platform, the customer was able to efficiently manage global clinical trials, improving collaboration across teams and reducing the overall time to market. The cloud-based system provided real-time data visibility, streamlining operations and ensuring that all stakeholders had access to up-to-date trial information, which is essential for maintaining inspection readiness and compliance. Additionally, the platform accelerated patient recruitment and improved operational workflows. The integration of digital tools for data capture, sharing, and storage enhanced overall trial efficiency, while ensuring regulatory requirements were met. This allowed the customer to focus more on advancing clinical research rather than operational complexities.
