Customer Overview
Founded in 1923, the institute is at the forefront of radiology evolution, representing more than 41,000 diagnostic and interventional radiologists, radiation oncologists, nuclear medicine physicians and medical physicists.
Challenges
The customer faced significant challenges with outdated in-house legacy systems that lacked the necessary integration capabilities for clinical trial oversight and data management. These legacy systems created silos of information, preventing efficient communication between different departments and making it difficult to have a unified view of clinical trial data. The absence of centralized systems led to issues related to data integrity, as information was often inconsistent and prone to errors. Compliance was another major concern, with the outdated technology struggling to keep up with evolving regulatory standards. Moreover, the old systems were becoming increasingly costly to maintain due to technology depreciation and frequent updates required to meet industry demands. As a result, the customer faced high maintenance costs and operational inefficiencies. To address these growing concerns and ensure smoother trial execution, the customer recognized the urgent need to transition to a centralized cloud-based solution that could reduce time and costs, improve trial efficiency, and optimize overall trial operations.
Solution
To tackle these challenges, the customer partnered with Cloudbyz to develop a centralized eClinical application and establish seamless integrations with multiple systems in their existing landscape. Cloudbyz implemented a solution that integrated Medidata Rave, RSS, Triad, Quic, and Sitecore systems, ensuring smooth connectivity and data flow across different platforms. The solution also included robust features such as CTMS, eTMF, Randomization, Patient Registration, User Registration, DDSI Portal, Site & Sponsor Portal, Unblinding Portal, and Safety Reporting, making the entire clinical trial process more transparent and manageable. Key EDC functionalities leveraged in this solution included eCRFs, Query Management, Source Data Review & Verification, and Patient Registration, which enhanced the overall collection and management of clinical data. The deployment of these integrated systems modernized the customer's trial management, ensured higher levels of data accuracy and compliance, and eliminated the need for multiple disconnected systems.
Benefits
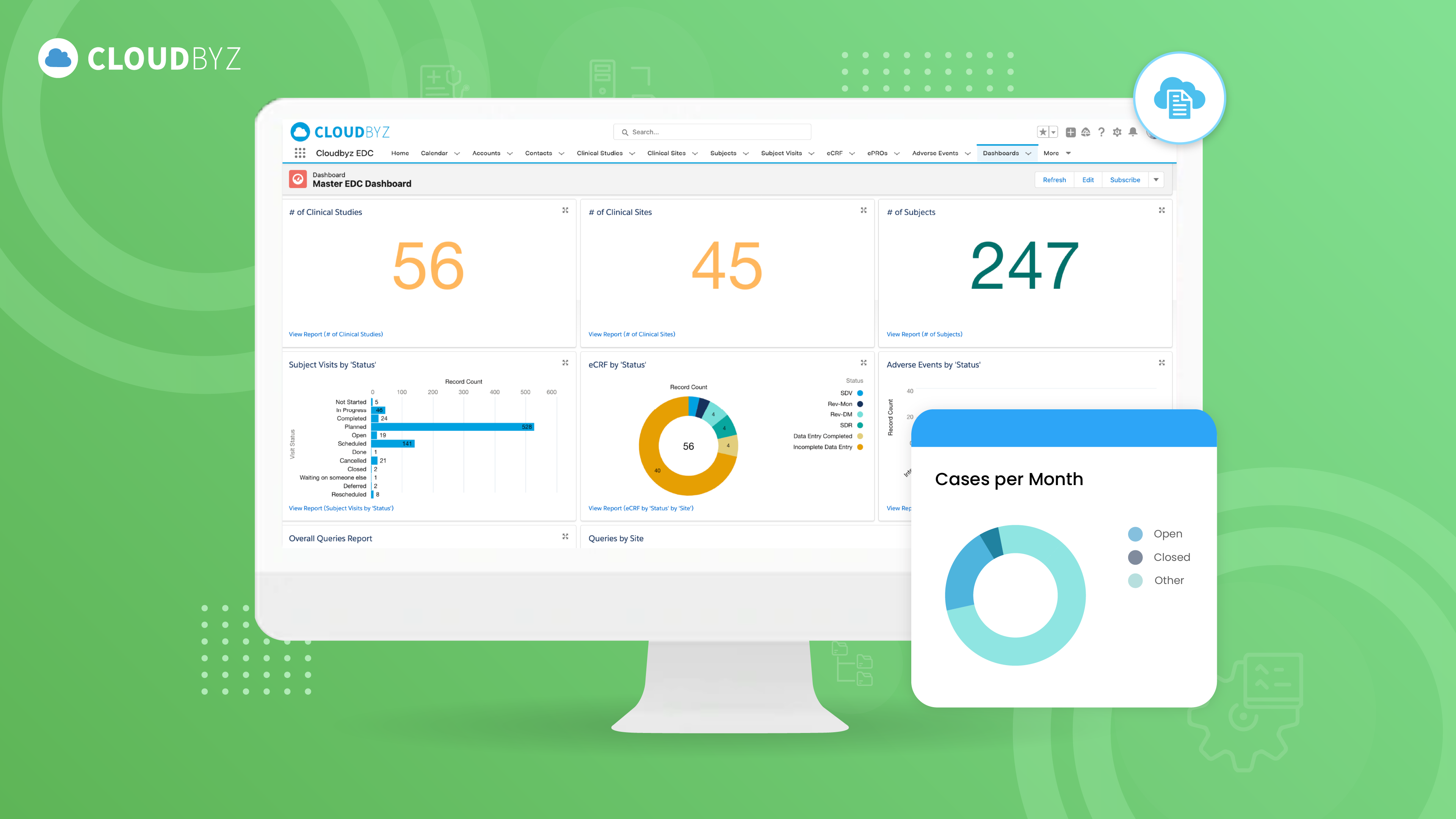
The adoption of Cloudbyz’s EDC system led to a substantial reduction in data collection errors and provided a significant increase in efficiency for the customer. By centralizing and automating the clinical trial data collection process, the customer was able to streamline workflows and enhance the accuracy of trial data. The real-time data capture capabilities of Cloudbyz EDC allowed for quicker, more informed decision-making, as stakeholders could access up-to-date trial information whenever needed. The system’s robust query management and real-time notifications further ensured that data discrepancies were identified and resolved promptly, reducing trial delays. Moreover, the adoption of Cloudbyz CTMS and eTMF led to a substantial reduction in operational errors and provided a significant increase in efficiency for the customer. The streamlined document management processes significantly reduced the time spent on regulatory submissions, ensuring that all documentation was inspection-ready. The expedited trial processes also optimized resource utilization, saving both time and costs. With these enhancements, the customer was able to run more efficient, compliant, and patient-centric trials, which improved collaboration between stakeholders and ultimately contributed to better clinical outcomes.