Request a demo specialized to your need.
In the dynamic world of biotechnology, clinical operations sit at the heart of translating scientific innovation into life-saving therapies. Yet, as biotechs grow, clinical operations often evolve organically — resulting in fragmented processes, siloed systems, and limited visibility. To scale efficiently and ensure regulatory excellence, organizations need a clear blueprint that connects strategy, people, process, data, and technology.
That blueprint is a Clinical Operations Business Capability Map — a structured framework that defines what a biotech organization must be able to do to execute its clinical research mission effectively.
Why a Capability Map Matters
A business capability map helps biotech organizations:
-
Align business and technology strategy: It connects high-level goals (like faster study start-up or inspection readiness) to specific capabilities and systems.
-
Enable digital transformation: It highlights gaps where automation, unification, or AI can add measurable value.
-
Drive scalability and compliance: It defines clear ownership, processes, and data structures for consistent quality and GxP compliance.
In essence, a capability map becomes the foundation for your clinical operations roadmap — ensuring that investments in CTMS, eTMF, EDC, or Safety systems directly support business outcomes.
The Capability Layers of Clinical Operations
1. Strategic & Governance Capabilities
At the top layer, governance and strategic alignment ensure that every clinical program ties back to the organization’s R&D vision.
Capabilities such as Clinical Strategy & Portfolio Management, Governance & Oversight, and Risk & Compliance Management establish frameworks for ethical conduct, regulatory adherence (GCP, ICH E6(R3), 21 CFR Part 11), and continuous improvement.
| Level 1 Capability | Level 2 Capabilities | Description |
|---|---|---|
| Clinical Strategy & Portfolio Management | Clinical portfolio planning, asset prioritization, study roadmap management | Aligns research programs and studies to the corporate pipeline and R&D objectives. |
| Clinical Governance & Oversight | Clinical steering committee management, policy & SOP governance, audit readiness | Establishes frameworks for oversight, quality assurance, and compliance. |
| Risk & Compliance Management | GCP, ICH E6(R3), GDPR, 21 CFR Part 11 compliance, CAPA management | Ensures adherence to global regulatory and ethical standards. |
2. Study Planning & Start-Up
Before a trial begins, planning and start-up activities determine its operational success.
Key capabilities include Protocol Design, Feasibility & Site Selection, Regulatory Submissions, and Site Contracting & Activation — all essential for compressing study timelines and ensuring regulatory readiness.
Modern eClinical platforms can digitize these processes, reducing startup time by weeks and improving collaboration between sponsors, CROs, and sites.
| Level 1 Capability | Level 2 Capabilities | Description |
|---|---|---|
| Study Feasibility & Design | Protocol design, study budgeting, site feasibility & selection | Involves scientific design, operational feasibility, and resource estimation. |
| Regulatory & Ethics Submissions | IRB/IEC submission, country regulatory submission, document package creation | Manages regulatory documentation, submissions, and approvals. |
| Site Contracting & Activation | Site negotiation, budgeting, contract management, site onboarding | Ensures site readiness with contractual and operational enablement. |
3. Study Execution & Monitoring
Once a study is live, operational excellence becomes critical.
Capabilities such as Site Management, Monitoring, Patient Recruitment, and Clinical Supply Management ensure that trials stay on track.
The integration of AI and risk-based monitoring (RBM) tools enables proactive oversight, helping CRAs and project managers identify deviations before they escalate.
| Level 1 Capability | Level 2 Capabilities | Description |
|---|---|---|
| Site Management & Monitoring | Site visit scheduling, remote monitoring, CRA activity tracking | Coordinates CRA activities and ensures site compliance and data integrity. |
| Patient Recruitment & Retention | Recruitment planning, patient engagement, diversity management | Ensures timely enrollment and retention strategies for study participants. |
| Data Collection & Source Verification | EDC, eCOA, ePRO, RBM, SDV & SDR | Manages digital data capture and monitoring to maintain data quality. |
| Clinical Supply & Logistics | IMP supply management, randomization, drug accountability | Ensures investigational product availability and logistics tracking. |
4. Clinical Data & Document Management
Data and document integrity are the backbone of compliant clinical operations.
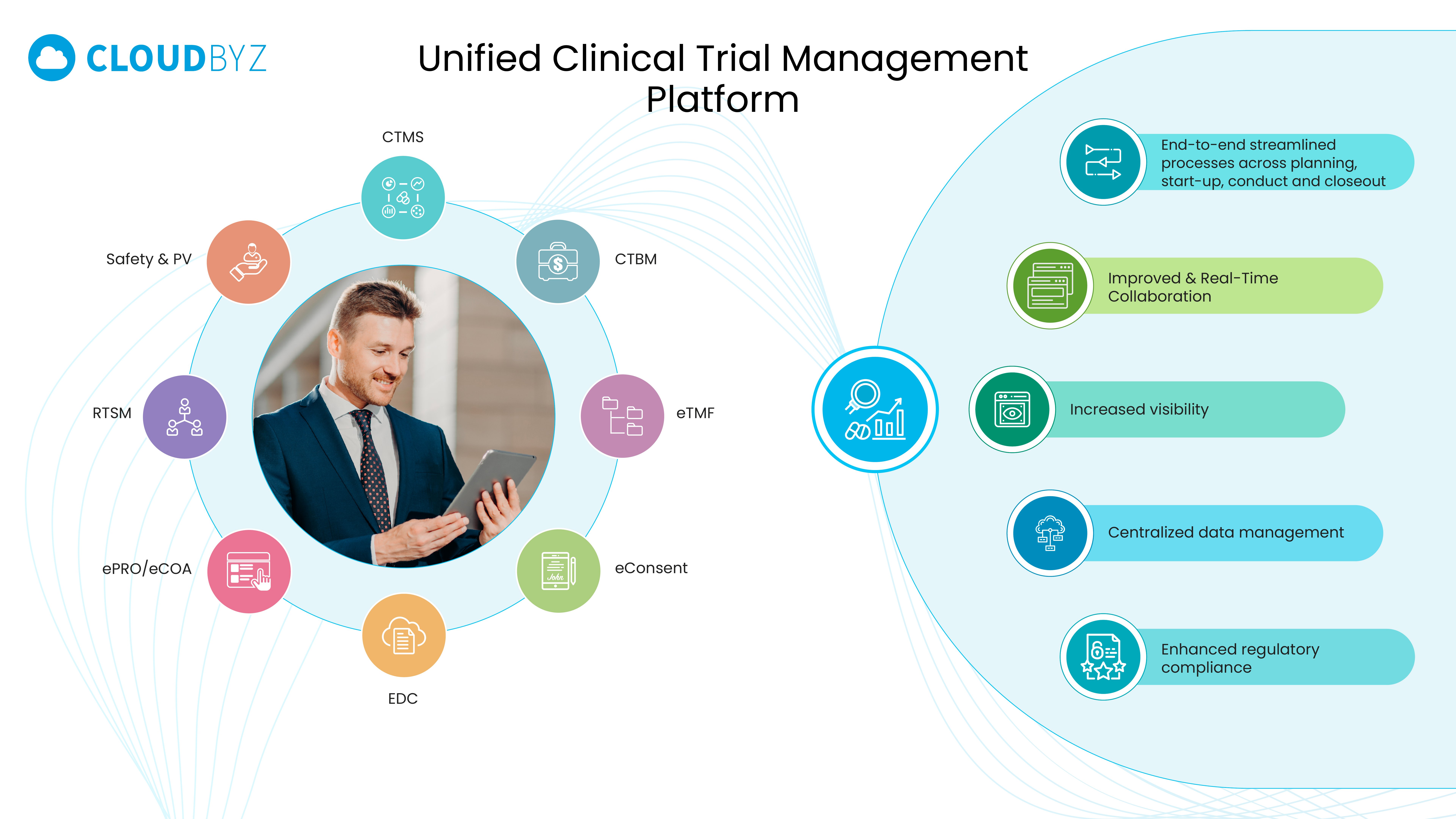
Capabilities like eTMF Management, Clinical Data Management (CDM), and Data Integration & Analytics create a single source of truth across studies.
Cloud-native solutions — such as Cloudbyz eTMF and EDC — unify workflows and automate metadata management, ensuring inspection readiness and faster data lock.
| Level 1 Capability | Level 2 Capabilities | Description |
|---|---|---|
| Trial Master File (TMF) Management | eTMF setup, document indexing, QC, and archival | Enables end-to-end electronic document lifecycle management. |
| Clinical Data Management (CDM) | eCRF design, edit checks, query management, database lock | Governs all aspects of clinical data collection, validation, and cleaning. |
| Clinical Data Integration & Analytics | Data warehousing, clinical insights, AI analytics, dashboards | Supports real-time insights through integrated analytics and dashboards. |
5. Safety & Pharmacovigilance
Patient safety is non-negotiable.
Capabilities such as Case Processing, Signal Detection, and Regulatory Reporting help biotechs capture, assess, and report adverse events in compliance with global standards.
Integrating safety systems with CTMS and EDC ensures seamless data flow from source to submission — reducing manual handoffs and improving traceability.
| Level 1 Capability | Level 2 Capabilities | Description |
|---|---|---|
| Safety Case Processing | AE/SAE intake, triage, MedDRA coding, narrative generation | Manages adverse event capture and medical review. |
| Safety Signal Detection & Risk Management | Signal analysis, aggregate reporting, risk mitigation planning | Proactively identifies safety trends and ensures mitigation. |
| Regulatory Reporting & Submissions | E2B(R3) gateway submissions, PSUR/DSUR preparation | Automates and manages regulatory reporting across geographies. |
6. Financial & Vendor Management
Clinical operations cannot run without financial control and vendor oversight.
Capabilities like Budgeting & Forecasting, Site Payments, and Vendor Oversight bring transparency and accountability.
Automation through solutions such as Cloudbyz CTFM enables milestone-based payments, real-time budget visibility, and accurate accrual forecasting — strengthening sponsor-site relationships.
| Level 1 Capability | Level 2 Capabilities | Description |
|---|---|---|
| Clinical Trial Budgeting & Forecasting | Budget templates, forecasting, cost control | Tracks study budgets and financial health. |
| Site Payments Management | Milestone tracking, payment triggers, disbursement automation | Ensures timely and transparent site payments. |
| Vendor Oversight & Contracts | CRO, labs, logistics vendor management | Monitors vendor SLAs, performance, and contractual compliance. |
7. Reporting, Analytics & Intelligence
Visibility drives smarter decisions.
Operational reporting and dashboards deliver real-time insights into study progress, while Regulatory Intelligence and AI Predictive Analytics help organizations plan proactively.
With connected data across CTMS, eTMF, and EDC, leaders can move from retrospective reporting to predictive intelligence.
| Level 1 Capability | Level 2 Capabilities | Description |
|---|---|---|
| Operational Reporting | KPIs, milestone tracking, site performance dashboards | Real-time visibility into study progress and risks. |
| Regulatory Intelligence & Insights | Global regulatory intelligence, country startup timelines | Supports decision-making with regulatory and operational benchmarks. |
| AI & Predictive Analytics | Risk-based monitoring, patient prediction models, AI agents | Leverages AI to enhance efficiency and decision quality. |
8. Cross-Functional & Enabling Capabilities
Quality, collaboration, and master data management form the connective tissue of clinical operations.
Capabilities like Quality & Audit Management, Collaboration & Communication, and Master Data Management ensure consistency and reliability across studies and systems.
| Level 1 Capability | Level 2 Capabilities | Description |
|---|---|---|
| Quality & Audit Management | QMS, audit trail, inspection readiness | Ensures quality assurance and inspection readiness. |
| Collaboration & Communication | Sponsor-CRO-site collaboration, workflow automation | Facilitates cross-functional communication and collaboration. |
| Master Data & Integration Management | Site, investigator, study, and subject master data | Maintains unified master data across systems. |
9. Technology Enablement Layer
Finally, every capability relies on a strong digital foundation.
From CTMS, eTMF, and Safety systems to AI-driven analytics and workflow automation — technology enables execution at scale.
Salesforce-native solutions like Cloudbyz Unified eClinical Platform empower biotechs to digitize, automate, and integrate operations across the full clinical lifecycle.
| Category | Example Solutions / Tools |
|---|---|
| CTMS | Cloudbyz CTMS |
| eTMF | Cloudbyz eTMF |
| EDC / eCOA / ePRO | Cloudbyz EDC |
| Safety | Cloudbyz Safety & PV |
| Financials | Cloudbyz CTFM |
| AI / Analytics | Salesforce Einstein, Tableau, AWS AI, Cloudbyz AI Agents |
Bringing It All Together
A well-defined Clinical Operations Capability Map is more than a process diagram — it’s a strategic lens for decision-making. It helps leaders:
-
Prioritize technology investments that matter most.
-
Identify capability gaps and maturity opportunities.
-
Build a roadmap toward unified, data-driven clinical excellence.
In the biotech industry, where agility and compliance must coexist, capability-led transformation is the key to sustainable growth.
Conclusion
As the industry continues to evolve toward AI-powered, unified digital operations, biotech organizations that define and mature their clinical capabilities will lead the next wave of innovation.
A capability map provides the foundation — a shared language between business and IT — to transform vision into execution.
Cloudbyz helps biotech companies accelerate this journey through a unified eClinical platform built natively on Salesforce, enabling end-to-end automation, collaboration, and real-time insights across clinical development.
Subscribe to our Newsletter

