Request a demo specialized to your need.
Budgeting in clinical trials doesn’t need to be a source of tension. With the right tools, it becomes a foundation for smoother collaboration, higher efficiency, and stronger site relationships.
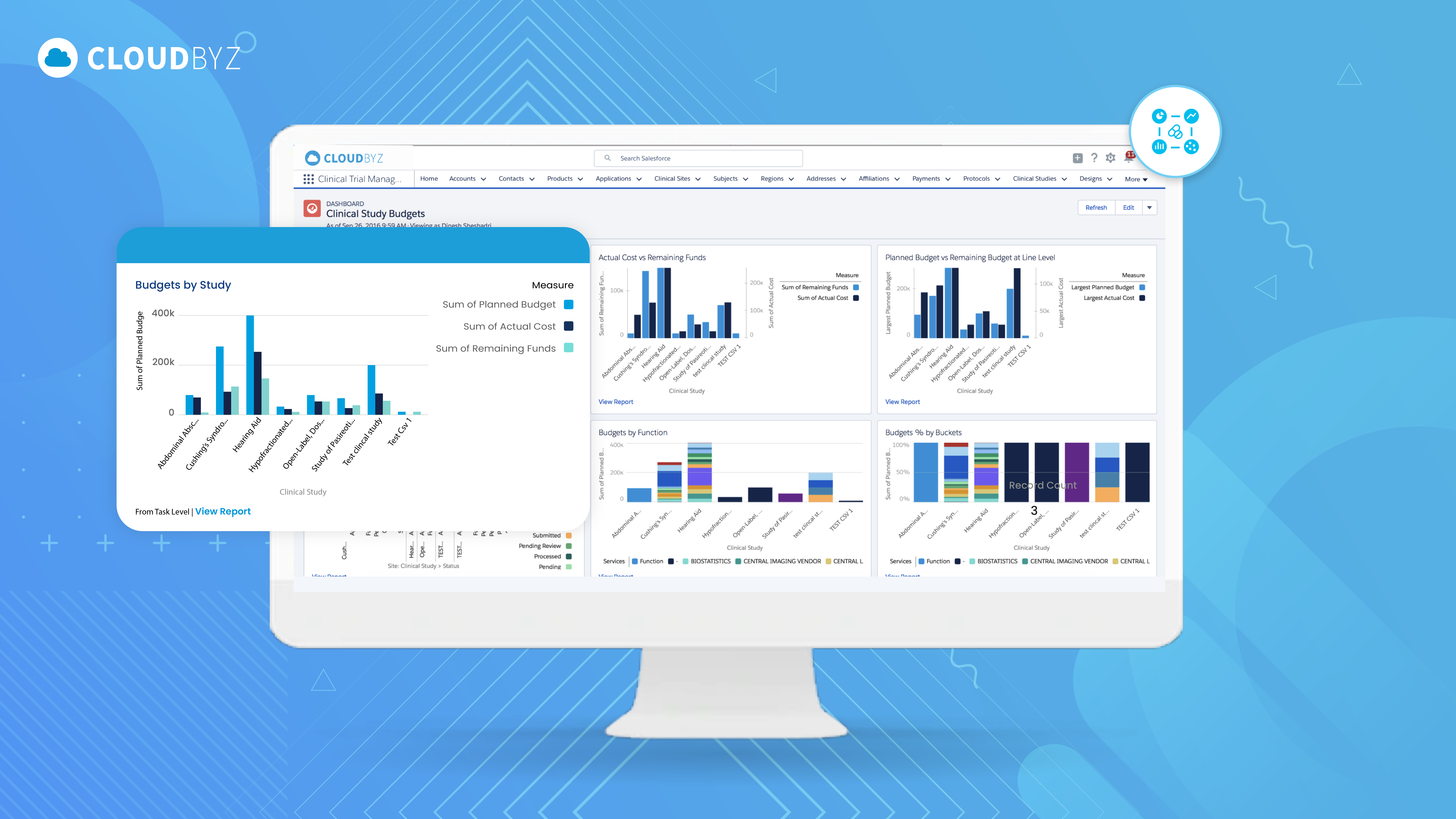
Budget negotiations in clinical trials often feel more like a maze than a well-structured conversation. Whether you're a sponsor trying to forecast trial costs, a CRO navigating coordination complexities, or a site managing tight resources, the financial side of clinical research can quickly become a source of frustration and friction.
The traditional approach to budgeting—reliant on spreadsheets, assumptions, and limited visibility—leads to misalignment, delays, and strained relationships among stakeholders. However, modern solutions like Cloudbyz Clinical Trial Financial Management (CTFM) are transforming the budgeting landscape by bringing structure, speed, and transparency to the forefront.
The Challenge: Misalignment Across Stakeholders
Sponsors: Seeking Accuracy and Accountability
Sponsors are under pressure to control costs while ensuring patient recruitment and retention. Without visibility into actual site-level performance or real-time financial data, many are left managing budgets based on outdated or overly generalized assumptions. This leads to under- or over-budgeting, which in turn affects financial forecasting and project viability.
CROs: Bridging Gaps with Limited Tools
CROs are expected to align sponsor goals with site realities, but often operate with fragmented data and manual processes. Managing different spreadsheets, negotiating budgets with sites, and tracking milestones without automation makes their role unnecessarily complex.
Sites: Waiting in the Dark
Sites frequently lack clarity on how budgets are structured and when payments will arrive. This opacity in invoicing and delayed reimbursements strains relationships, reduces trust, and can even lead to site dropout—especially in resource-constrained geographies.
The Solution: Cloudbyz CTFM—Clarity and Control at Every Level
Cloudbyz CTFM is designed to address these stakeholder-specific pain points with a unified, intelligent platform. Here's how:
Template-Based Budget Creation
Cloudbyz leverages protocol-aligned templates to rapidly generate detailed budgets linked to visit schedules. These templates eliminate guesswork and bring consistency across trials, while reducing time spent in negotiation cycles.
Standardized Library of Procedures, Tasks, and Rates
The platform comes with a built-in library of standard operating procedures (SOPs), common tasks, and rates that reflect both Fair Market Value (FMV) and Standard of Care (SOC) benchmarks. This ensures realistic, region-specific budgeting that sites can trust and sponsors can validate.
Intelligent Payment Triggers at Visit & Procedure Level
Unlike legacy systems that wait for visit completion alone, Cloudbyz enables payment triggers down to the procedure level within each visit. This granular milestone tracking means invoices reflect actual work done—nothing more, nothing less—giving both sites and sponsors confidence in every transaction.
Transparent Invoicing & Payment Reconciliation
CTFM creates an open channel of communication between sites, CROs, and sponsors by offering visibility into payment status. Sites know exactly when payments are triggered, processed, and completed—eliminating the “black box” effect and reducing administrative follow-ups.
Full Integration with Clinical Systems
Cloudbyz integrates seamlessly with CTMS, EDC, and eTMF platforms, enabling accurate tracking of both planned and actual spend. This makes variance analysis straightforward and ensures audit-ready financial documentation throughout the trial lifecycle.
Why Transparency Matters Now More Than Ever
As trials become more decentralized and patient-centric, the pressure on financial clarity increases. Sites need quicker reimbursements. Sponsors need clean forecasting data. CROs need better operational control.
With Cloudbyz CTFM, stakeholders no longer need to choose between speed and precision. The platform's data-driven, automation-first approach ensures:
-
Faster study startup through template-based planning
-
Increased trust with sites via real-time payment visibility
-
Better sponsor oversight with analytics-rich dashboards
-
Reduced back-and-forth during budget negotiations
Cloudbyz Clinical Trial Financial Management solution helps organizations gain visibility, insight, and control across clinical trials and studies. The solution helps in accelerating study budget management with a high level of accuracy with faster cycle time. Cloudbyz CTFM enhances decision-making, maximizes resource utilization, and increases operational efficiency.
To know more about Cloudbyz CTFM Solution contact info@cloudbyz.com
Subscribe to our Newsletter

