Request a demo specialized to your need.
Monitoring of clinical trials is a critical activity required to be carried out during the entire lifecycle of the trial to validate that it is conducted as per the approved protocol. Monitoring should also uphold the GCP requirements to ensure the integrity of trial data, safety, and rights of subjects.
The following are the major risks and occurrences that trial monitoring sets out to identify and recommend corrective actions:
- Data inaccuracy in clinical trial results due to improper maintenance of trial record and data.
- Unknown side effects of investigational drugs causing patient discomfort in participation.
- Impact on patient safety due to serious adverse events (hospitalization or even death), with lack of monitoring leading to higher patient risk.
- Equality of patient benefit may be unattended without proper monitoring.
- The inconvenience of traveling incurring additional costs and post-admission glitches on patient safety.
Careful and frequent monitoring can ensure early detection of risks during the course of a clinical trial. Traditionally, monitoring was done through full-scale Source Data Verification (SDV) that involved manual verification of all collected data from source documents and records. In due course of time, four algorithm dependent approaches like random SDV approach, declining SDV approach, three-tiered SDV approach, and mixed approaches were implemented to reduce the manual effort and time involved in SDV.
While these techniques were an improvement to the traditional SDV process, the associated manual effort, time and costs were still significantly high, galvanizing the collective effort of industry and regulatory participants to look out for alternative approaches.
The following are the major challenges of 100% SDV approach to trial monitoring:
- Since the SDV performed was primarily a manual review process, monitoring is error-prone and was able to provide 85% review accuracy at best.
- The manual SDV process involves repeated reviews adding to resource cost as well as significant time investment.
- The SDV is not effective and efficient in dealing with the following factors
- Transcription errors within the source document,
- Misrepresentation of the subject information or irrelevant data
- If an anomaly is being observed in the clinical trial process, it is challenging for the regulators to capture that particular information alone and suggest the CAPA for the same. Hence, the monitoring process is repeated again making the entire cycle tedious and costly.
- Managing and maintaining different versions of SDV documentation would be painful for the regulators as well as Sponsors and CROs involved in clinical research.
The Transition towards Risk-based Quality Management
The FDA has recognized the potentials of risk-based monitoring to improve quality in all phases throughout the course of study trials. The most significant changes in ICH E6 (R2) have been implemented to insist on Sponsors and CROs to adopt a risk-based approach for study execution. The modern automated RBM solutions facilitate centralized, off-site monitoring with the objective to minimize the risks associated with study trials coupled with the implementation of GCP, SDR and Planning metrics. The modern approach would also eliminate the need to undergo the repeated process, by capturing the particular information of the risk assessment which needs corrective action for subsequent reviews. The system captures all the information and the workflow process which can be validated by the regulators easily along with time and date stamp.
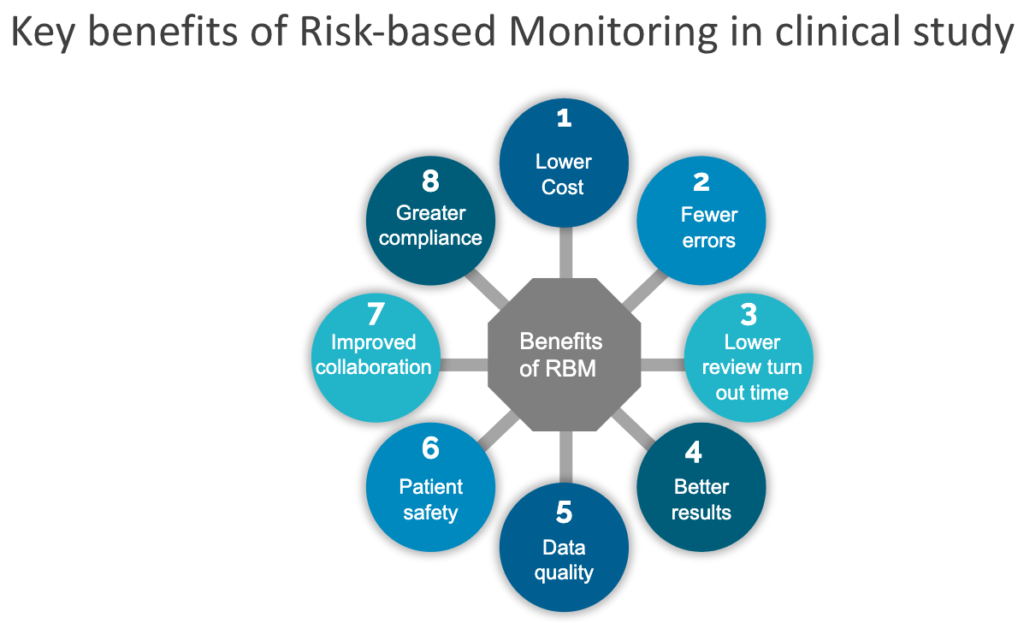
Risk-based monitoring has the potential to dramatically reduce the costs, time and errors without any adverse impact on the overall trial quality.
 Risk-Based Monitoring using Cloudbyz RBM Solution
Risk-Based Monitoring using Cloudbyz RBM Solution
Cloud computing and advanced software-as-a-service platforms have led to breakthroughs in RBM application. One of the key benefits leveraging technologies is efficient centralized risk-based monitoring and analysis of clinical trial data in real-time.
To enable the industry to follow the regulators’ general guidance, Cloudbyz has developed a risk-based clinical trial monitoring solution, with the objective of enabling organizations to improve data quality and patient safety in their clinical trials. Cloudbyz RBM solution can be adopted by organizations of any size and adapted to any type or phase of a clinical trial.
- Risk Assessment: The guidance for GCP, ICH E6, and ISO14155 require a structured approach to assess risks in terms of identifying the source, evaluating the risks in terms of its impact on trial data and patient safety, create risk mitigation plans and track corrective measures till the risks are eliminated or mitigated. Cloudbyz RBM solution enables study monitors and team to configure risk assessment through various processes like risk detection, risk control, risk communication, risk evaluation, risk review, and risk reporting.
- Data integration: In most clinical trials, lack of structured integration strategies has led to inaccuracy of data. Cloudbyz RBM features can be integrated with ePRO, eCRF, IVRS/IRT to provide real-time visibility on clinical trial data and performance metrics. The feature also helps in seamless data flow throughout the course of the clinical trial process.
- Easy to configure: The trials can be configured and managed securely by an authorized administrator using this solution. The Cloudbyz RBM solution is natively built on a Salesforce Cloud platform which is flexible and configurable to tailor the solution to meet the study requirements.
- Scalability: The solution is built natively on the Salesforce platform and is highly scalable and extensible to deal with a large amount of data as the study progresses and the number of studies increases.
- Collaboration: Cloudbyz RBM solution supports cross-functional study team management to review risks centrally through the seamless end-to-end workflow.
- Real-time data visibility: Data retrieved from discrete EDC and Trial management systems without a centralized approach is tedious leading to inconsistency and latency. Cloudbyz RBM solution provides real-time visibility to all the trial data and has open APIs to be able to easily integrate with external EDC systems and provide 360-degree visibility to trial performance. It also provides the ability to set thresholds on key metrics and can send instant notification to the right stakeholders when those limits are breached.
- Reporting: Clinical trial reports should be free from ambiguity, well organized and easy to review. The report should provide a clear explanation of the clinical design, protocols, conduct of the study, sufficient interpretation of patient data, including the demographic and baseline data, and details of analytical methods. The Cloudbyz RBM solution provides targeted, actionable, sustained, meaningful reporting that enables trial teams to take action on the issues detected with configurable, point-and-click reports and dashboards.
- Compliance: Cloudbyz RBM solution is fully compliant with FDA 21 CFR part 11 regulations. Electronically captured records are saved automatically in a secured database. The captured records and documents are maintained with strong access controls and granular audit trail.
5 Best practices – Considerations for accounting for variations in costs
References:
http://www.appliedclinicaltrialsonline.com/basics-clinical-trial-centralized-monitoring
https://www.clinicalleader.com/doc/new-fda-guidance-answers-questions-on-rbm-use-0001
Subscribe to our Newsletter

