Request a demo specialized to your need.
Biotech start-ups are focused on bringing innovative therapies to market as quickly as possible. However, the path from drug discovery to commercialization is long and complex, with clinical trials representing a significant bottleneck in the process. Traditional clinical trial management methods can be time-consuming, expensive, and error-prone, which can delay the launch of potentially life-saving therapies. In this blog post, we will explore how biotech start-ups can accelerate the launch of their therapies by leveraging a unified clinical trial management platform.
Biotechnology start-ups face unique challenges when it comes to bringing new therapies to market, as they often have limited resources, smaller budgets, and less experience in the industry. One way they can accelerate their path to the market is by leveraging a unified clinical trial management platform.

What is a Unified Clinical Trial Management Platform?
A unified clinical trial management platform is a cloud-based software solution that integrates all aspects of clinical trial management into a single, centralized platform. This includes everything from study design and patient recruitment to data collection and analysis. By bringing together all the different components of clinical trial management, a unified platform streamlines the entire process, making it faster, more efficient, and more cost-effective.
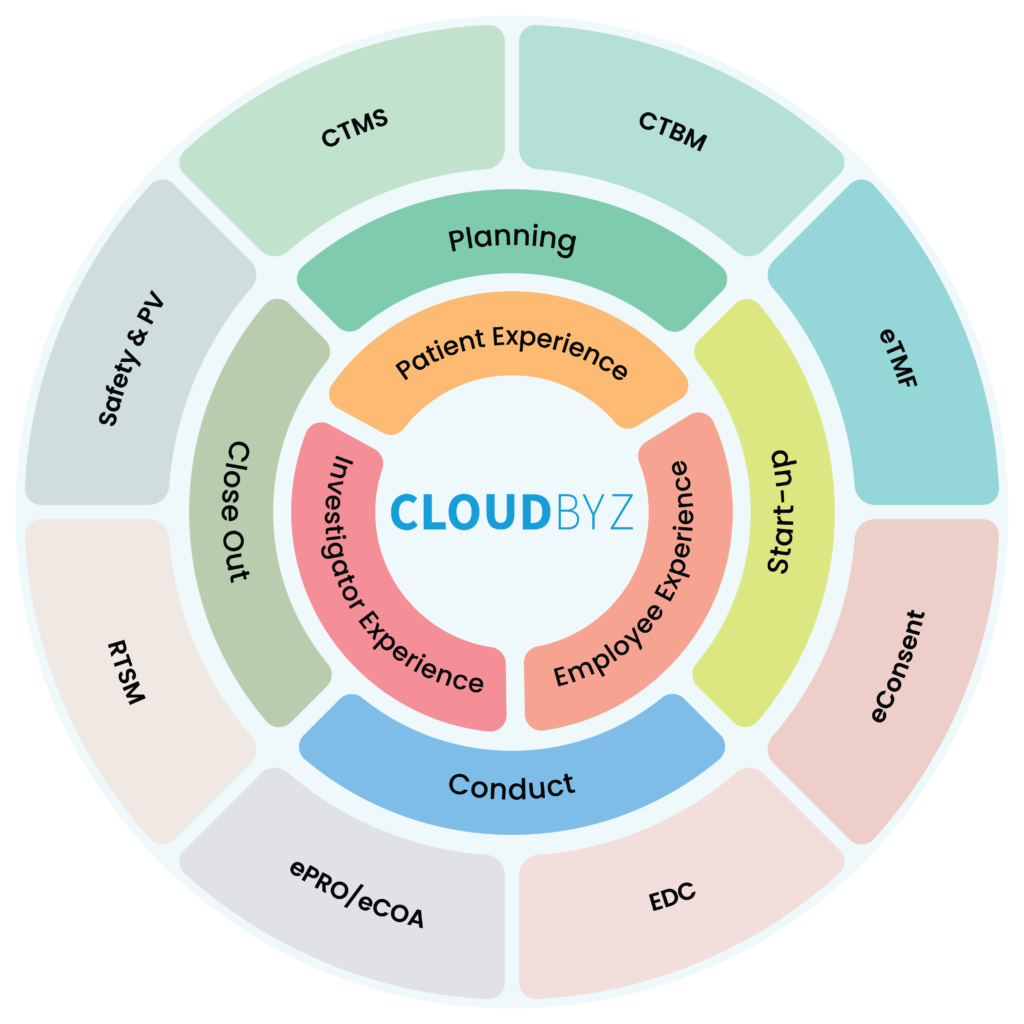
Here are some ways in which a unified clinical trial management platform can benefit biotechnology start-ups:
- Faster Study Start-up: With a unified platform, biotech start-ups can design their studies, identify potential sites, and initiate study start-up processes in a fraction of the time it takes with traditional methods. This means they can get their trials up and running faster, which is critical for bringing therapies to market quickly.
- Streamlined processes: A unified clinical trial management platform can streamline processes and improve efficiency across all stages of clinical trials. This can help biotechnology start-ups to reduce the time to market and accelerate the development of their therapies.
- Improved collaboration: A unified clinical trial management platform can facilitate collaboration between different teams working on the same trial. This can help to reduce errors and inconsistencies in data and streamline communication between team members.
- Increased visibility: A unified clinical trial management platform can provide real-time visibility into trial data and progress. This can help biotechnology start-ups to identify potential issues or delays early on and take corrective action before they become major problems.
- Better data management: Clinical trial data is complex and requires careful management and analysis. A unified clinical trial platform can simplify data collection, provide real-time data visualization, and enable data analysis and interpretation. It provides a single source of truth for all trial data, reducing the risk of errors and inconsistencies. Real-time data analysis and visualization enable biotech start-ups to make data-driven decisions, accelerating the development of new therapies and reducing time to market.
- Enhanced regulatory compliance: Compliance with regulatory requirements is critical for biotech start-ups looking to bring new therapies to market. A unified clinical trial platform can help ensure compliance by providing a centralized platform for regulatory reporting and documentation. It enables real-time monitoring of trial data, simplifies adverse event reporting, and provides audit trails to demonstrate compliance. This reduces the risk of regulatory delays and ensures that biotech start-ups can bring new therapies to market as quickly as possible.
- Advanced analytics: A unified clinical trial management platform can provide advanced analytics capabilities, which can help biotechnology start-ups to gain insights into trial data and identify patterns and trends. This can help to improve trial design and inform future decision-making.
- Cost Savings: Clinical trials are expensive, and biotech start-ups often have limited resources. A unified clinical trial platform can help reduce the costs associated with clinical trials by streamlining administrative tasks, improving patient recruitment, and enabling data-driven decision-making. It also reduces the risk of errors and delays, which can result in significant cost savings in the long run. Cost savings enable biotech start-ups to invest more resources in developing new therapies, bringing them to market faster and improving patient outcomes.
Overall, a unified clinical trial management platform can help biotechnology start-ups to accelerate their path to market by improving efficiency, collaboration, visibility, data management, compliance, and analytics. By leveraging such a platform, biotechnology start-ups can reduce the time and cost of clinical trials, improve the quality of trial data, and increase the chances of getting their therapies approved by regulatory agencies.
Cloudbyz Unified Clinical Trial Management (CTMS) is a comprehensive and integrated solution designed to streamline clinical trial operations. Built on the Salesforce cloud platform, our CTMS provides real-time visibility and analytics across study planning, budgeting, start-up, study management, and close-out. With features like automated workflows, centralized data management, and seamless collaboration, Cloudbyz CTMS can help you achieve greater efficiency, compliance, and quality in your clinical operations. Contact us today to learn more about how Cloudbyz CTMS can help your organization optimize its clinical trial management processes.
To know more about the Cloudbyz Unified Clinical Trial Management Solution contact info@cloudbyz.com
Subscribe to our Newsletter

