Request a demo specialized to your need.
Subscribe to our Newsletter

Dinesh
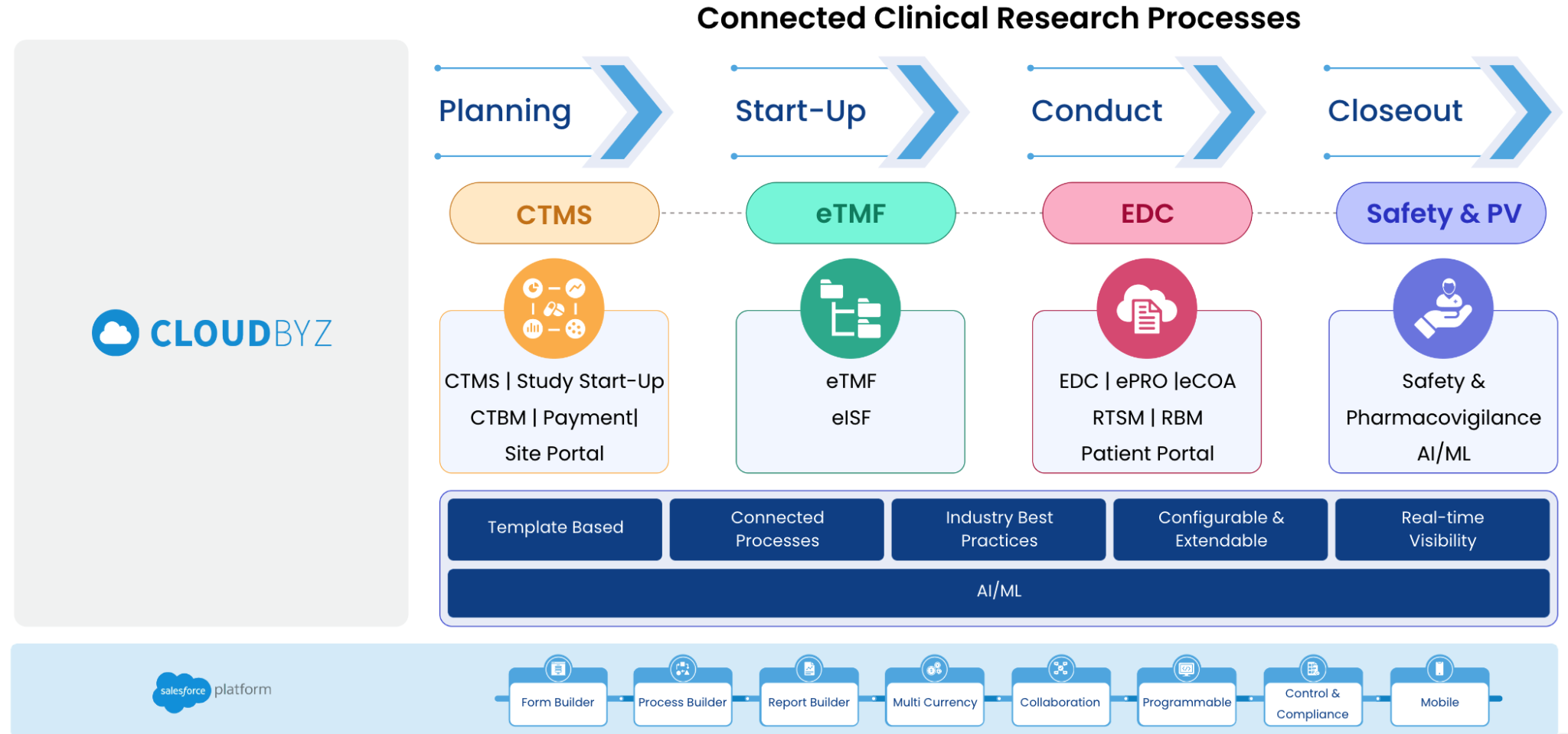
Clinical trials are complex and resource-intensive endeavors, and as such, there is an increasing need to streamline clinical trial operations and increase operational efficiency. A unified platform that integrates multiple clinical trial management capabilities can be a game-changer in improving operational efficiency in clinical trials. In this analysis report, we will explore how integrating CTMS, Clinical Trials Financials, eTMF, EDC, and Safety in one unified platform can help improve clinical trial operational efficiency.
Unified Clinical Trial Management refers to the integration of all clinical trial processes into a single platform, allowing for streamlined and efficient management of study protocols, data collection, patient recruitment, regulatory compliance, and overall trial operations. This approach offers real-time visibility into trial progress, improved data quality, and reduced operational costs.
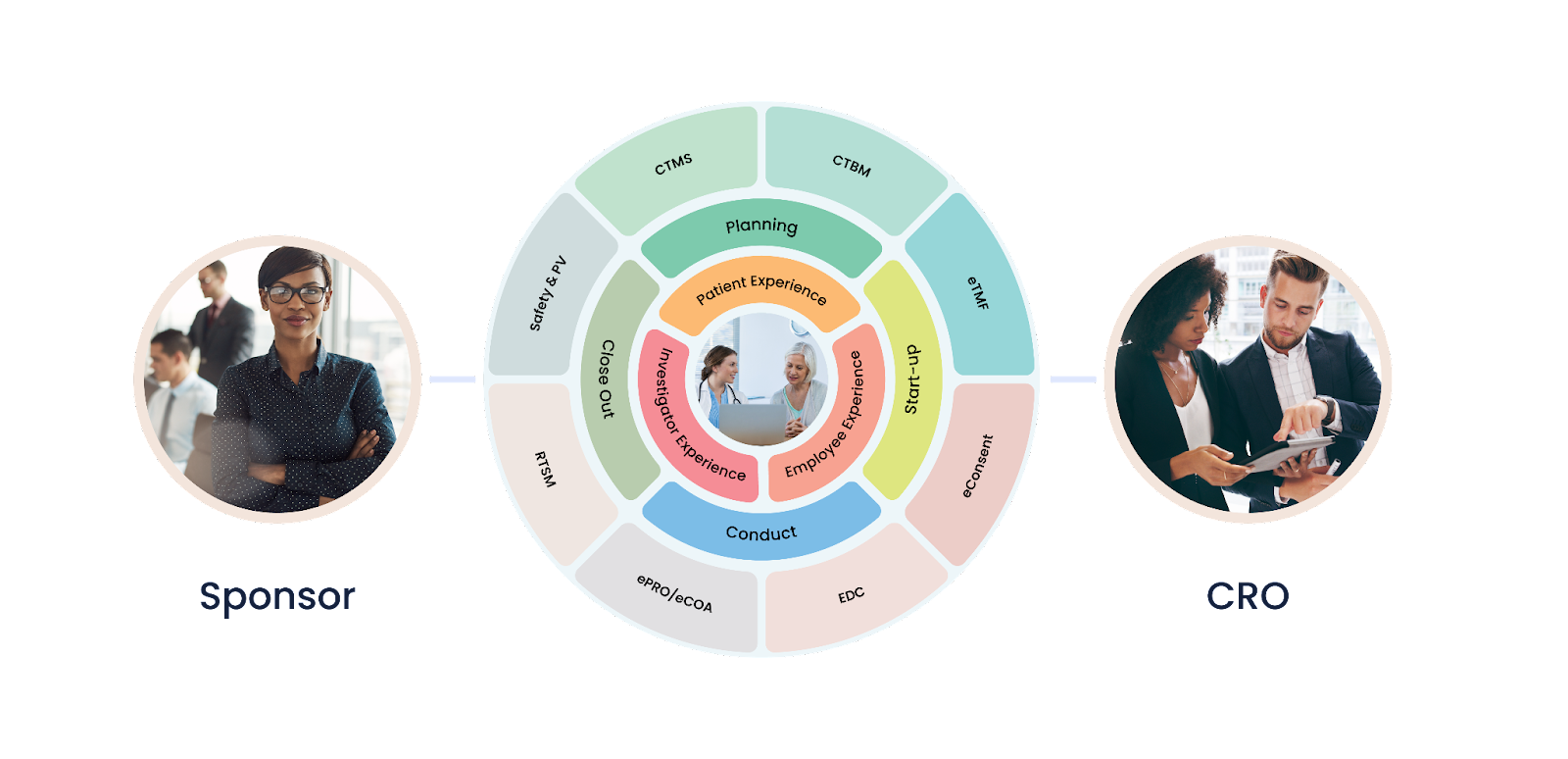
Integrating CTMS, Clinical Trials Financials, eTMF, EDC, and Safety into one unified platform can help accelerate clinical trials in several ways. Here are some of the key ways that a unified platform can speed up the clinical trial process:
Integrating CTMS, Clinical Trials Financials, eTMF, EDC, and Safety into one unified platform can help streamline the study setup and initiation process. With a unified platform, study teams can more easily manage the budget, timeline, and vendor selection process, leading to faster study initiation.
Integrating EDC into a unified platform can enable faster data capture and cleaning by reducing the need for manual data entry. This can help to reduce data entry errors and speed up the data cleaning process. A unified platform can also integrate data from other sources, such as electronic medical records, to further accelerate the data capture process.
A unified platform can provide real-time monitoring and oversight of study progress, allowing study teams to identify issues and take corrective action quickly. This can help to reduce the risk of study delays and improve the overall efficiency of the clinical trial.
A unified platform can facilitate streamlined collaboration and communication between different study teams and stakeholders, enabling faster decision-making and problem resolution. This can help to reduce delays and accelerate the clinical trial process.
Integrating eTMF into a unified platform can help ensure regulatory compliance by providing a centralized repository for all essential documents and records. This can reduce the risk of non-compliance and delays due to missing or incomplete documentation, speeding up the clinical trial process.
A unified platform can help study teams to more efficiently manage study budgets, expenses, and vendor payments, leading to more cost-effective clinical trials. By providing a single source of truth for financial data, a unified platform can also reduce the risk of financial errors and discrepancies.
In conclusion, integrating CTMS, Clinical Trials Financials, eTMF, EDC, and Safety into one unified platform can help accelerate the clinical trial process by streamlining study setup and initiation, enabling faster data capture and cleaning, providing real-time monitoring and oversight, improving collaboration and communication, ensuring regulatory compliance, and enabling more efficient financial management. By leveraging the benefits of a unified platform, study teams can accelerate clinical trial timelines and improve the efficiency of the entire clinical trial process.
Cloudbyz Unified Clinical Trial Management (CTMS) is a comprehensive, integrated solution to streamline clinical trial operations. Built on the Salesforce cloud platform, our CTMS provides real-time visibility and analytics across study planning, budgeting, start-up, study management, and close-out. Cloudbyz CTMS can help you achieve greater efficiency, compliance, and quality in your clinical operations with features like automated workflows, centralized data management, and seamless collaboration. Contact us today to learn how Cloudbyz CTMS can help your organization optimize its clinical trial management processes.
To know more about the Cloudbyz Unified Clinical Trial Management Solution contact info@cloudbyz.com
Subscribe to our Newsletter

ISO 9001:2015 and ISO 27001:2013 Certified